Take-home Points
|
 |
|
Bios Dr. Sadda is director of Artificial Intelligence and Imaging Research at the Doheny Eye Institute and professor of ophthalmology at the University of California Los Angeles David Geffen School of Medicine. DISCLOSURES: Dr. Sadda serves as a consultant to Amgen, AbbVie/Allergan, Genentech/Roche, Oxurion, Novartis, Regeneron, Iveric, 4D Molecular Therapeutics, Centervue, Heidelberg Engineering, Optos, Merck, Apellis, Astellas, Nanoscope, Gyroscope Therapeutics, Janssen and Pfizer; receives speaker fees from Carl Zeiss Meditec, Nidek, Novartis and Optos; and received research instruments from Nidek, Topcon, Heidelberg, Carl Zeiss Meditec, Optos and Centervue. |
Diabetic retinopathy remains a leading cause of blindness among working-age individuals worldwide.1 As retina specialists, a key component of our management of these patients is identification of diabetic lesions and staging of the severity of DR.
Our current approach to staging derives from the system originally proposed by the Airlie House investigators in 1968.2 This system was based on comparing the severity and extent of specific diabetic lesions in various fields relative to standardized color photographs.
A total of seven 30-degree fields were chosen with a focus in the posterior pole to include the macula, optic nerve, and surrounding regions as these were thought to be most relevant for vision and to be involved early on in the disease process.
Limitations of ETDRS
The Airlie House system was further modified by the Diabetic Retinopathy Study and Early Treatment of Diabetic Retinopathy Study (ETDRS) investigators to develop a DR severity scale (DRSS) that paralleled the progression of the disease and predicted the risk for progression to proliferative diabetic retinopathy and vision loss.3 The ETDRS-derived DRSS has been shown to be highly reproducible and has served as the backbone for clinical trials in DR over the last several decades.4 Indeed, the reliability of this scale has allowed us to use steps of progression on this scale as an endpoint for regulatory approval of pharmacotherapeutic agents.5,6
Despite the enormous success and impact of the ETDRS-based DRSS, the system does have several important limitations, in large part due to the constraints of the imaging technology available at the time the system was developed; namely, small-field color film photography. As a result, the system was ultimately qualitative and categorical rather than quantitative.
While a higher DRSS level indicated more advanced disease, the distance between steps was not necessarily linear. A qualitative system is also relatively imprecise. The ETDRS scale features multiple levels and is based on repeatability; a two-step progression was deemed to be meaningful.7
Desire for quantitative analyses
However, in this era of pharmacotherapeutics, where a regression of some DR lesions may be observed, a quantitative approach—that is, one based on precise number or area of lesions—may be preferred.8
Indeed, quantitative analyses of specific lesions, such as microaneurysms, has revealed that microaneurysm turnover may represent an additional risk factor for progression of DR.9 Another limitation of the DRSS is that, while it has been invaluable in clinical research, it has proven difficult to fully translate to clinical practice. Comparison to standardized photographs and assessments of multiple fields is simply impractical in the context of a busy practice. Many simplifications, including the International Clinical disease severity scale for DR (ICDR),10 have been proposed for routine clinical use, but these yield further loss of precision and still aren’t consistently used by clinicians.
Perhaps the biggest limitation of the ETDRS-derived DRSS, however, is that it’s based on a presumed representative sample of the retina that was easily accessible by standard fundus cameras. DR, however, can impact the entire retina. Advances in retinal imaging, in particular ultra-widefield (UWF) technology, has made photographic capture of the peripheral retina feasible. Whereas the ETDRS seven standard fields only covered about 30 percent of the total retinal surface area, modern UWF devices, especially with the use of steered images, can capture 90 percent or more of the retinal surface area.
Notably, Paolo S. Silva, MD, and colleagues observed that more than 60 percent of diabetic lesions (hemorrhages, microaneurysms, intraretinal microvascular abnormalities, neovascularization) were located outside the seven ETDRS fields.11
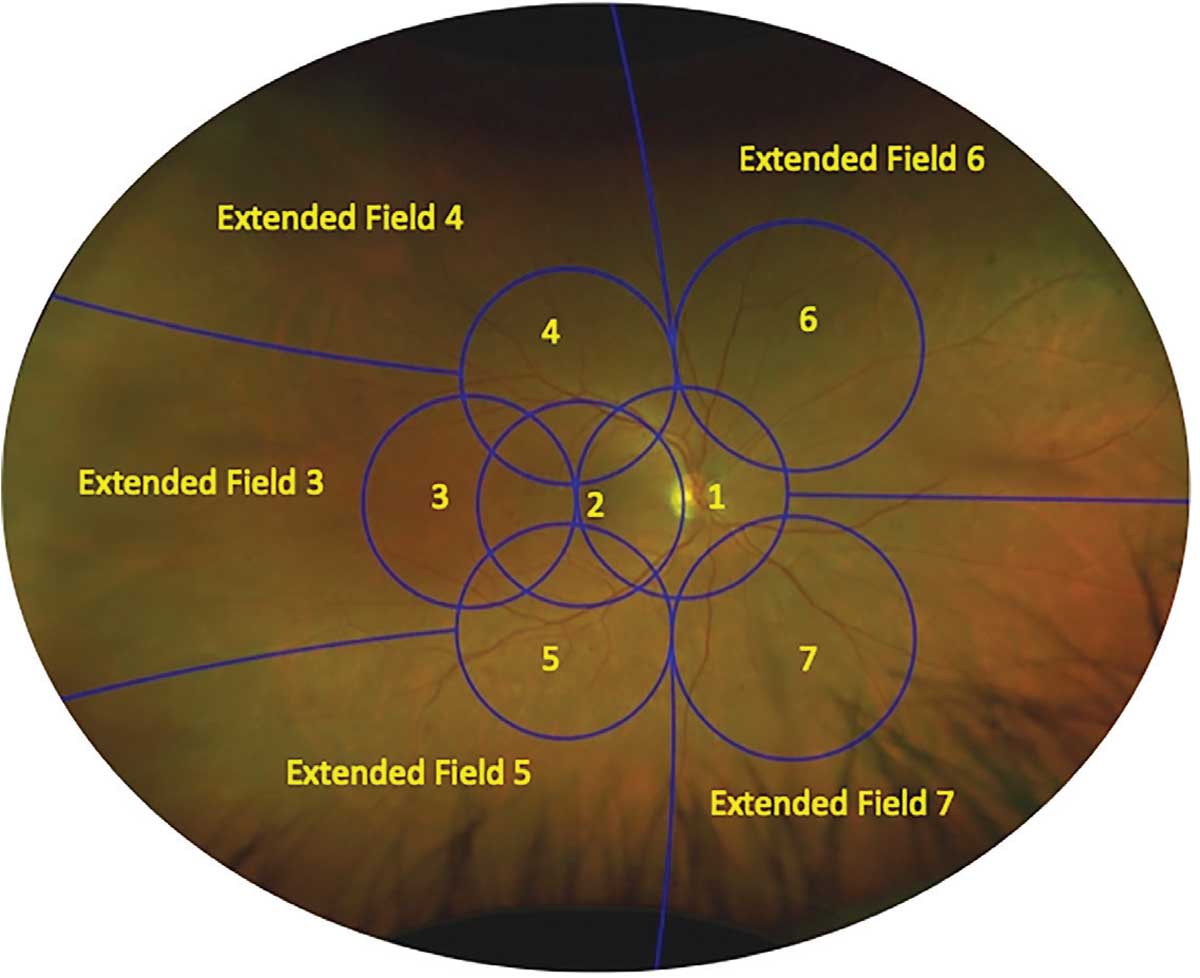 |
| Figure 1. Ultra-widefield Optos pseudocolor image of the right eye of a patient with moderate nonproliferative diabetic retinopathy shows the seven standard Early Treatment Diabetic Retinopathy Study fields and the corresponding extended peripheral lesions. In this case, there are more extensive diabetic retinopathy lesions in extended field 6 compared to the corresponding ETDRS field 6, and thus this eye would be deemed to have predominantly peripheral lesions. |
Peripheral lesions and DR severity
Thus, the key question for consideration here is this: Is the assessment of these DR lesions beyond the seven standard fields of clinical importance? Dr. Silva and colleagues observed that if the peripheral lesions were considered, a higher severity level of DR would have been selected in about 10 percent of their cases.11 This suggests that failure to consider peripheral DR lesions on the whole may lead to an underestimation of the DR severity level.
Neovascularization elsewhere (NVE) lesions or areas of preretinal hemorrhage (PRH) outside the seven standard fields would seem to be of particular concern, because they could lead to more rapid and severe vision loss if they’re undetected and consequently not monitored or treated appropriately.
 |
If we accept that peripheral lesions in DR could potentially be of clinical relevance, how do we actually assess them in the context of our current approach to staging DR? Dr. Silva and colleagues proposed five peripheral extended fields adjacent to ETDRS fields 3 to 7, within which the severity of DR lesions could be assessed.12
However, there are no reference standard photographs of lesion severity for these larger extended fields. In the absence of such references, they proposed comparing lesion severity in the peripheral extended fields with the corresponding ETDRS field (Figure 1). If any of the five extended fields demonstrated more DR lesions compared to its corresponding ETDRS field, they deemed the eye to contain predominantly peripheral lesions (PPL).13 The presence of PPL is a frequent finding in eyes with DR. In a study of more than 1,400 eyes with DR, we observed that 37 percent of eyes had PPL, and more than 30 percent PPL was observed at all ETDRS DRSS levels from mild nonproliferative DR to PDR.14
In a subsequent, small (n=109 eyes) longitudinal study, Dr. Silva and colleagues demonstrated that DR eyes with PPL had about a four times greater risk for progression to PDR than eyes without PPL.13 This is a striking and potentially transformative result, given that peripheral lesions weren’t even considered in the ETDRS system.
Validating the value of peripheral lesions
As a result, the DRCR Retina Network initiated Protocol AA to validate whether evaluation of the retinal far periphery on UWF images improves our ability to assess DR and predict rates of DR worsening over time as compared with evaluation only of the area within the seven standard ETDRS fields.15 Results are expected soon, and if the previous observations are confirmed, one can expect a significant evolution of our current approach to staging DR.
Protocol AA will hopefully be able to answer other important questions. Among them: Is the current method (i.e., single-field determination of PPL) for assessing the retinal periphery the best approach for staging DR? For example, should we be considering the entire periphery vs. the posterior pole (i.e., a global assessment) rather than individual fields? Also, should we be considering only the number of lesions? What about the size or surface area of lesions? We have shown that the classification of the peripheral retinopathy can change depending on the method used.16
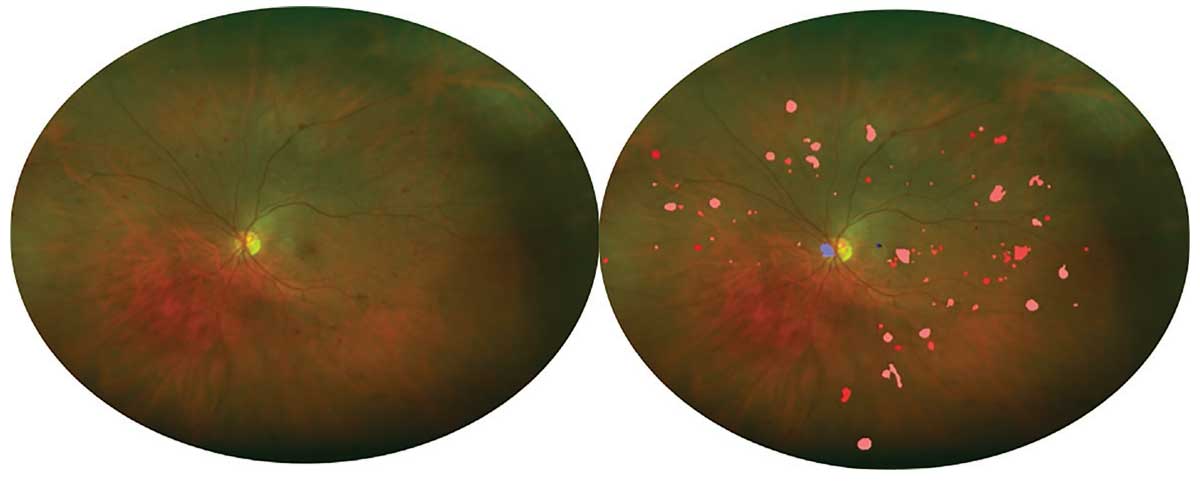 |
| Figure 2. Left: Ultra-widefield Optos pseudocolor image of an eye with severe nonproliferative diabetic retinopathy. Right: Automated detection of DR lesions using a deep-learning algorithm. The number and distribution of lesions may be quantified. |
Potential of AI
Perhaps an even more fundamental question is: Why should we be limited to categorical or qualitative methods from a previous era that were necessitated by the constraints of film-based photography? In this era of digital imaging and artificial intelligence (AI), it may be possible to quantify diabetic lesions throughout the fundus including the retinal periphery.
There are currently two Food and Drug Administration-cleared AI-based technologies for automated detection of referral-warranted DR.17,18 Both of these systems, however, were designed for standard field fundus cameras. We applied one of these systems (EyeArt) to UWF Optos pseudocolor images and observed good performance for detecting referral-warranted DR, even without optimizing the algorithms for UWF images.19
This bodes well for future AI-based screening using UWF images, but significantly highlights the possibility that UWF images may be combined with AI/deep learning to quantify the location, number and size of all DR lesions throughout the retina in the eyes of our diabetic patients (Figure 2, above).
Using this quantitative approach to assessing DR in the entire retina, we demonstrated a correlation between the number of DR lesions and the ETDRS DRSS.20 If Protocol AA confirms the importance of more peripheral DR lesions, one may be able to incorporate this into a quantitative staging system by
 |
assigning a greater weight to a DR lesion based on its distance from the center of the fundus.
Bottom line
Advances in UWF imaging have enabled the assessment of DR lesions throughout the fundus, including the retinal periphery. Preliminary studies suggest that DR eyes with predominantly peripheral lesions may be at higher risk for progression to PDR, though this requires confirmation by the pending results from Protocol AA. Combining UWF imaging with automated deep-learning tools may make it possible to quantify all DR lesions throughout the eye. This may open the door for a new quantitative and precise staging system that may transform our approach for diagnosing and monitoring our patients with diabetic retinopathy. RS
REFERENCES
1. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. 2021;128:1580-1591.
2. Milton RC, Ganley JP, Lynk RH. Variability in grading diabetic retinopathy from stereo fundus photographs: comparison of physician and lay readers. Br J Ophthalmol. 1977;61:192-201.
3. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786-806.
4. Solomon SD, Goldberg MF. ETDRS Grading of diabetic retinopathy: Still the gold standard? Ophthalmic Res. 2019;62:190-195.
5. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946-955.
6. Zhang J, Strauss EC. Sensitive detection of therapeutic efficacy with the ETDRS diabetic retinopathy severity scale. Clin Ophthalmol. 2020;14:4385-4393.
7. Klein R, Klein BE, Moss SE. How many steps of progression of diabetic retinopathy are meaningful? The Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2001;119:547-553.
8. Ashraf M, Cavallerano JD, Sun JK, Silva PS, Aiello LP. Ultrawide field imaging in diabetic retinopathy: Exploring the role of quantitative metrics. J Clin Med. 2021;10:3300. doi:10.3390/jcm10153300
9. Pappuru RKR, Ribeiro L, Lobo C, Alves D, Cunha-Vaz J. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br J Ophthalmol. 2019;103:222-226.
10. Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1182.
11. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: Distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587-2595.
12. Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465-2472.
13. Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949-956.
14. Verma A, Alagorie AR, Ramasamy K, et al. Distribution of peripheral lesions identified by mydriatic ultra-wide field fundus imaging in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:725-733.
15. Aiello LP, Odia I, Glassman AR, et al for the Diabetic Retinopathy Clinical Research Network. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65-73.
16. Sears CM, Nittala MG, Jayadev C, et al. Comparison of subjective assessment and precise quantitative assessment of lesion distribution in diabetic retinopathy. JAMA Ophthalmol. 2018;136:365-371.
17. Abramoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
18. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4:e2134254. doi:10.1001/jamanetworkopen.2021.34254
19. Wang K, Jayadev C, Nittala MG, et al. Automated detection of diabetic retinopathy lesions on ultrawidefield pseudocolour images. Acta Ophthalmol. 2018;96:e168-e173.
20. Sadda SR, Nittala MG, Taweebanjongsin W, et al. Quantitative assessment of the severity of diabetic retinopathy. Am J Ophthalmol. 2020;218:342-352.



