Take-home points
|
 |
|
Bios DISCLOSURES: Dr. Patel has no relevant relationships to disclose. Dr. Klufas reports relationships with Genentech/Roche, Regeneron Pharmaceuticals, Allergan/AbbVie and RegenexBio. |
Last year, the Food and Drug Administration approved the port-delivery system with ranibizumab, now called Susvimo (ranibizumab injection, Genentech/Roche) 100 mg/mL, for intravitreal use via ocular implant for the treatment of neovascular age-related macular degeneration that responded to at least two anti-VEGF injections, regardless of anti-VEGF agent.
PDS is an innovative technology that aims to reduce treatment burden for both the patient and clinician. Direct-to-patient advertising and local news stories on the FDA approval of PDS have increased awareness of it, and more patients are inquiring about it. In this article, we discuss pearls for implementing PDS in your practice.
Who’s a good candidate for PDS?
Two populations of patients may be worthwhile for further discussions about PDS:
- Patients with a strong motivation to not have frequent injections or those who don’t like the injection procedure regardless of interval. This can be different from the patient’s and doctor’s perspectives. For instance, a patient getting injections every eight weeks may feel like their day is “shot” afterward and they would be willing to undergo PDS placement to have a procedure only twice a year.
- Patients who get injections every four to six weeks but aren’t totally compliant. Their noncompliance may be due to medical or social factors. From a physician and patient perspective, they may have better control of exudation on optical coherence tomography and better objective (or subjective) vision with more frequent therapy. Essentially, these are patients that we as physicians know do better with monthly therapy but, in reality, only get 10 or fewer injections in a typical 12-month period, as several real-world studies have shown.
Additional clinical characteristics to consider for ideal candidates
What’s more, aside from the treatment interval, you should consider additional clinical characteristics to determine if PDS is an acceptable alternative to traditional intravitreal injections. Ideal candidates have a few key characteristics that include:
- an appropriate age; the chance that a single implant will last the patient’s remaining lifetime is greater the older a patient is, although we don’t have firm data supporting this;
- a mobile conjunctiva that doesn’t appear thin in the superotemporal quadrant;
- no history of conjunctival incision; and
- a low chance of requiring an additional conjunctival incisional procedure, such as a glaucoma filtering procedure, in the next five to 10 years.
Informed consent, black-box warning
Additionally, the patient should be able to undergo the informed consent process and understand the need for perioperative visits as well as the risks, benefits and alternatives to the current standard of care-—i.e., continued intravitreal injections.
We should describe the black-box warning regarding an increased risk of endophthalmitis vs. monthly intravitreal injections to patients. Indeed, in the Archway trial, the risk of endophthalmitis was 1.6 percent (4/248), and all cases occurred more than one month after the initial surgery (days 57, 59, 161 and 282).1
As with any novel surgical device, our understanding of the potential long-term complications of PDS will evolve. We should discuss with patients specific risks such as implant dislocation and conjunctival erosion.
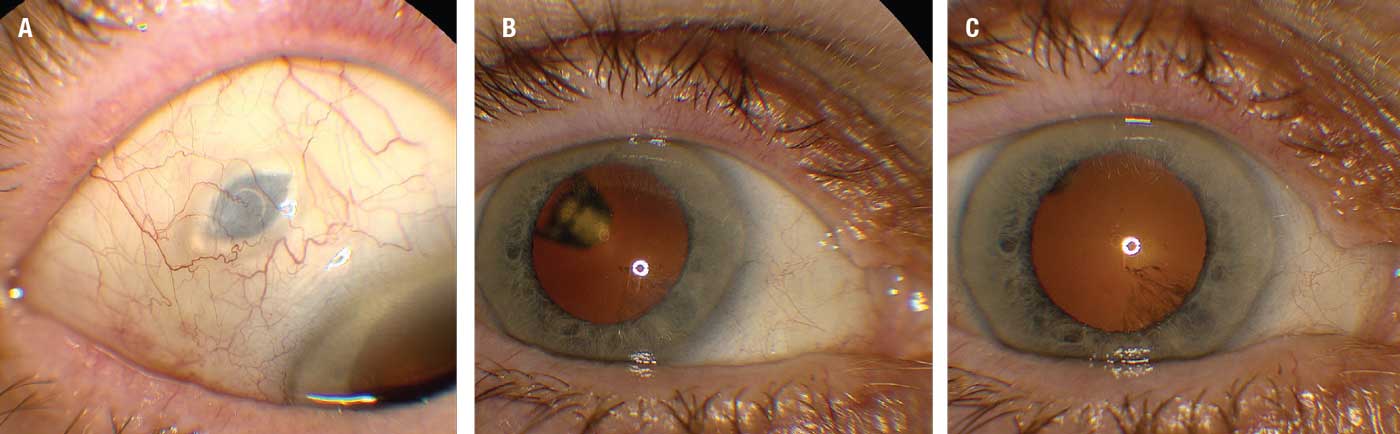 |
| Figure 1. External photographs of the port delivery system with ranibizumab implant (Susvimo) one month after insertion. A) The implant as it appears under the healed sclera. B) A view of the implant through the dilated pupil with the eye fixated temporally. C) A view through the pupil fixated centrally. (Courtesy Carl D. Regillo, MD) |
Potential red flags
Potential red flags for PDS implantation may be patients who are constantly switching insurances or thinking about changing their insurance. Nothing sounds like more of a nightmare than implanting a PDS successfully only to have the patient switch insurance and the new payer only allows off-label bevacizumab injections.
Furthermore, a patient may not be an ideal candidate if they’ve had a history of conjunctival procedures such as pterygium excision or glaucoma filtering procedures, given that the conjunctiva is such an important physiologic barrier to infection.
Surgical pearls for the PDS implant
 |
For a vitreoretinal surgeon, this isn’t a difficult procedure, but it is unique. It requires attention to detail to ensure long-term surgical success (Figure 2; watch video here). Genentech has several great resources to help ensure success when integrating PDS into your clinical practice, and a surgical device liaison will be in the operating room with you for the first few cases. What’s more, there are several enhancements in the implantation procedure that have led to improved outcomes since the Archway1 and Ladder2,3 trials.
Careful handling of the conjunctiva with non-toothed forceps and meticulous closure of the conjunctiva and Tenon’s capsule after the surgery are paramount to avoid conjunctival erosion of the device. Think like a glaucoma specialist with the conjunctiva and not a retina surgeon!
A traction suture, which we as retina specialists don’t routinely use during our bread-and-butter vitreoretinal procedures, can also be very helpful, even in the setting of a well-trained assistant available to rotate the eye to allow improved exposure of the superotemporal quadrant. If the periocular block isn’t complete, the patient can have a tendency to Bell’s up, thus making the superotemporal quadrant more difficult to visualize and work in.
Tips for sclerotomy
As with any surgical procedure, each step builds upon the previous one, so it’s important to be meticulous with each step to avoid a future problem. For example, when making the sclerotomy, use the fixed metal guide that comes with the implant so that the opening is approximately 3.5 mm.
 |
It’s paramount not to make the sclerotomy too large, because in the next step you’ll cauterize the choroid with an endolaser probe, which can lead to some retraction of the scleral tissue, thus enlarging the scleral incision slightly.
An incision that’s too large isn’t optimal for placement, but it’s correctable with a suture. We recommend recording your first few clinical cases and reviewing the surgical videos to improve upon any deficiencies in the first couple of cases.
Handling prolapsed vitreous
A vitrectomy isn’t part of the standard procedure for PDS. Rarely, upon creation of the 3.5-mm sclerotomy, prolapsed vitreous may emerge along the edges. Given that a vitrectomy pack is opened as part of this procedure to allow placement of an infusion line in the event of hypotony, the cutter can be used prior to inserting the device to remove any residual vitreous from the sclerotomy. Upon insertion of PDS, the vitreous typically retracts into the eye. It’s important to avoid a Wek-Cel vitrectomy.
Emphasize the need for post-op visits
As with any vitreoretinal procedure, standard postoperative visits should be planned at one day, one week and one month. It’s important to set patient expectations before implantation that there will be at least three postoperative visits, assuming no complications.
Additionally, I would counsel the patient who’s had a long history of intravitreal injections to expect there will be a slight decrease in vision for the first two to four weeks after the procedure while the eye heals.
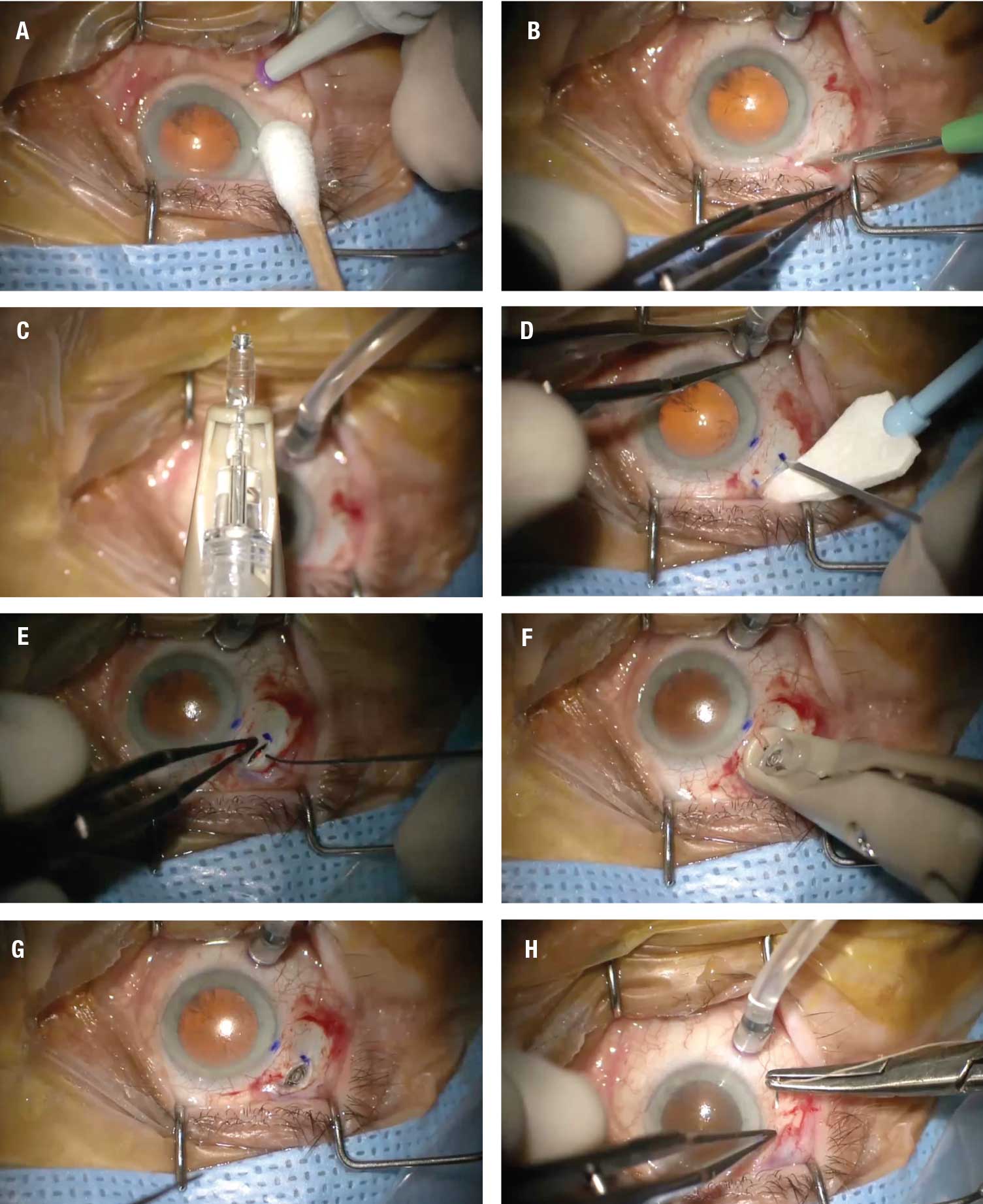 |
| Figure 2. Key steps in the placement of the port delivery system implant, as demonstrated in the video at bit.ly/RetSpecMag_2022_03 and embedded below: A) Placement of the infusion cannula inferotemporally. B) Creation of a 6-x-6-mm Tenon’s peritomy. C) Filling of the implant before insertion. D) Creation of the scleral dissection. E) Use of endolaser probe to photocoagulate the uvea. F) Insertion of device into the sclera. G) The device seated in the sclera. H) Closure of the conjunctiva. (Courtesy Carl D. Regillo, MD). Click image to enlarge. |
Initially, the standard plan is to monitor patients at the same interval at which they were receiving injections previously. For example, if a patient was receiving injections every six weeks, I would plan to monitor every six weeks postimplantation until the planned refill at six months. After the initial refill, I would try to extend the monitoring interval to 12 weeks.
Under ideal circumstances, a PDS patient would be seen four times a year, with two of those visits for refill/exchange. Monitoring might be more frequent if the other eye has high-risk nonexudative AMD or if the patient is functionally monocular with the PDS implanted eye.
Rescue intravitreal injections can be given with the PDS implanted in the inferotemporal quadrant if exudation persists. Indeed, in Archway, 1.6 percent of patients (4/248) received supplemental ranibizumab before the first refill-exchange procedure.1
Potential post-op complications
Educate the patient about potential warning symptoms, including red eyes, ocular discomfort or foreign-body sensations, which may be precursors to complications such as conjunctival erosion or retraction. It may also be helpful to educate other eye-care providers who may be caring for the patient to evaluate the superotemporal conjunctiva for potential signs of erosion if these symptoms arise.
Given the risk of endophthalmitis, increasing floaters, eye pain and/or increasing redness should also be reported at any time after the implant. I often have patients ask me about the outcomes of endophthalmitis with PDS, and from the Archway trial the cases treated with vitreous tap and intravitreal antibiotics, with or without irrigation of the PDS with antibiotic, generally did well and didn’t require device removal. However, outcomes after infectious endophthalmitis in PDS, as with postinjection endophthalmitis may be variable and outcomes after infectious endophthalmitis in PDS, as with postinjection endophthalmitis, may be variable and are driven by the causative infectious agent.
The refill procedure is probably the most foreign and most challenging part of the PDS therapy. It’s a bit more precise than a typical intravitreal injection and requires the refill exchange needle to be properly oriented to enter the PDS to allow refill exchange.
It’s important that no trapped air is in the refill exchange needle; an excess amount of drug is provided to account for this. A pair of low-magnification lighted loupes can help improve visualization of the device’s silicone septum to allow proper placement of the refill needle and also proper alignment. Without proper alignment, excessive force may be applied to the device and increase the risk of possible device dislocation. Twisting motions of the needle or other maneuvers aren’t necessary and can even bend the small 34-gauge needle. With magnification, proper placement and alignment, needle entry shouldn’t encounter significant resistance.
The tactile response of piercing the device’s fibrous capsule is also novel compared to the standard intravitreal injection. Don’t be surprised if it feels a bit different than piercing the sclera. It’s sometimes helpful to have a cotton-tip applicator in the other hand to stabilize the globe during the docking. Once the refill needle is docked, the most difficult part of the procedure is over, and depressing the plunger to allow the new drug in and exchange the old drug out is very similar to any other intravitreal procedure.
Bottom line
With a number of more durable neovascular AMD treatments on the horizon, along with the relatively recent approval of the PDS itself, patients will show greater interest to learn if they’re ideal candidates for PDS. Consider multiple factors to determine if a patient is a good candidate. That should include having a robust discussion of the risks and benefits of the operation. In patients who are ideal candidates, surgical preparation and collaboration with your local device liaison will be important to ensure success with the initial surgery and future refills. RS
REFERENCES
1. Holekamp NM, Campochiaro PA, Chang MA, et al. Archway randomized Phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;129:295-307.
2. Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: Results from the randomized Phase 2 Ladder clinical trial. Ophthalmology. 2019;126:1141-1154.
3. Khanani AM, Callanan D, Dreyer R, et al. End-of-study results for the Ladder Phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina. 2021;5:775-787.



