 |
| Richard Mark Kirkner, Editor |
The Port Delivery System with ranibizumab (PDS) has been the focus of much attention in the late summer. Phase III Archway trial data presented at the American Society of Retina Specialists showed the vast majority of patients—98.4 percent to be precise—went six months before needing any additional treatment.1
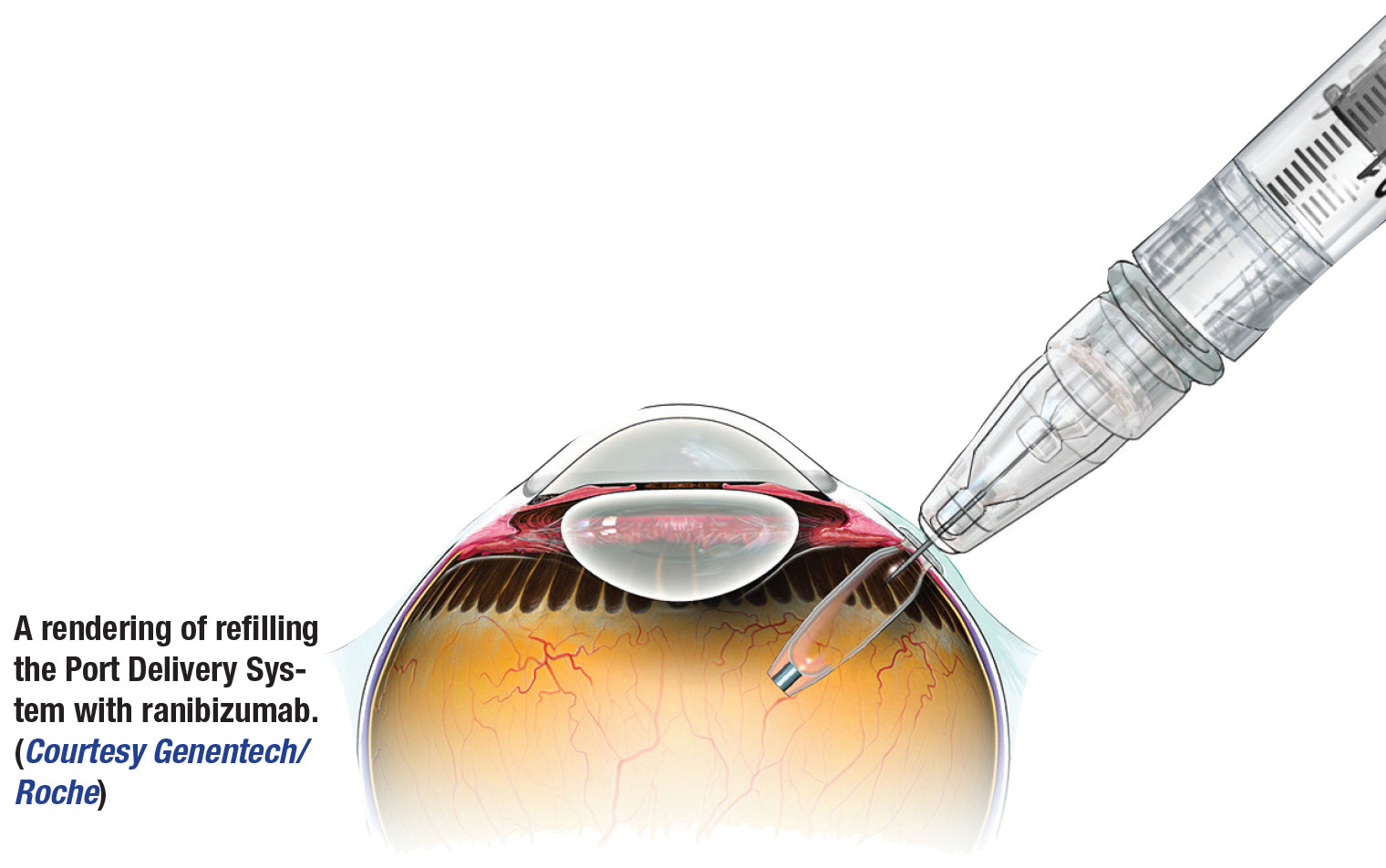
Some basics about PDS with ranibizumab: It’s a refillable eye implant that Genentech/Roche describes as the size of about a grain of rice. The manufacturer also emphasizes that the ranibizumab in the PDS is different from Lucentis used for intravitreal injection. The PDS is designed to continuously deliver a formulation of ranibizumab into the eye over a course of months and eliminate the need for more frequent intravitreal injections.
The Archway trial consists of 418 participants with neovascular age-related macular degeneration randomized to PDS with 100 mg/mL ranibizumab or intravitreal injections of 10 mg/mL Lucentis. The primary outcome measure was the mean change from baseline best-corrected visual acuity averaged over weeks 36 and 40. Data collection ended in March, with the study completion expected mid-spring next year.
Here, principal investigator Peter A. Campochiaro, MD, of the Wilmer Eye Institute at Johns Hopkins University in Baltimore, and who presented the Archway results at ASRS, answers questions about the trial and the PDS with ranibizumab itself.
 |
Q: Everyone understands the mechanism of ranibizumab well. Does the sustained release via PDS alter the bioavailablity of the drug in any way?
Yes, it makes the drug available continuously unlike intraocular injections which result in peaks and valleys. Depending on the frequency of intraocular injections, there are periods when there is no ranibizumab in the eye, whereas with the PDS ranibizumab is present in the eye continuously.
After implantation of the PDS or refill/exchange, the level of ranibizumab in the eye is greater than trough levels seen with monthly injections for 12 months. The amount of ranibizumab needed for efficacy varies somewhat among patients, but at six months after implantation the levels of ranibizumab in the eye are in the therapeutic range for almost all patients. That’s why refill/exchanges are done every six months.
 |
Q: How does this formulation differ from Lucentis?
It’s Lucentis, but the concentration is higher than that given by intraocular injection. In patients with wet AMD, we give 50 µl of a 10 mg/ml solution of Lucentis by intraocular injection which is a total dose of 0.5 mg. In the PDS the reservoir holds 20 µl and it’s filled with a 100 mg/ml solution of ranibizumab. So the total amount in the reservoir is 2 mg but it’s released slowly into
the eye.
Q: What’s involved with the implantation process?
It’s a very easy procedure, but it’s important to pay attention to a few important details.
It’s usually done under local anesthesia with sedation. A 27-gauge cannula is inserted and an infusion line is attached but not turned on. A conjunctival flap is dissected in the superotemporal quadrant taking care to dissect under Tenon’s capsule so that it remains attached to the conjunctiva. A radial incision is made in the horizontal meridian. Light cautery is applied to the episclera to obtain hemostasis, and then at 4 mm posterior to the limbus a 3.5-mm incision is made through the sclera to expose the pars plana.
The pars plana is treated with long-duration, overlapping burns of laser photocoagulation to cauterize all the blood vessels within it. One should check to make sure the infusion is off and then an MVR blade is used to penetrate the pars plana. The PDS is then inserted with a PDS-insertion tool and it’s tamped down to make sure the flange and septum protrude as little as possible above the scleral surface.
The conjunctiva, with Tenon’s capsule attached, is then closed, making sure to place two episcleral bites to secure it at the limbus. The limbal edge of the conjunctiva should actually overlap the peripheral edge of the cornea so that as it retracts, it settles down right at the limbus.
Q: What’s the most significant finding of the Archway trial?
The most significant finding is that, compared with monthly injections of ranibizumab, the PDS showed equivalent maintenance of visual acuity.
Q: What was the most surprising finding from the trial?
That 98 percent of patients in the PDS group didn’t require a supplemental injection prior to the first refill/exchange. This indicates excellent reliability in reaching the target duration. With any sustained delivery approach, high reliability is important to eliminate the need for extra visits for monitoring.
Q: Are there any secondary findings of significance?
In a patient-reported outcome survey, patients reported a strong preference for the PDS over intraocular injections.
Q: Were there any safety issues with the implantation and refill procedures?
There were four cases of endophthalmitis, three of which were related to conjunctival retraction, so appropriate management of the conjunctiva is important as emphasized earlier. One of these patients had permanent, severe vision loss, but the other three were effectively treated and vision returned to baseline.
Q: What’s the next readout we can expect from the trial?
Long-term outcomes will be reported after all patients complete the week 96-visit. RS
REFERENCE
1. Campochiaro P. Primary analysis results of the Phase 3 Archway trial of the port delivery system with ranibizumab (PDS) for patients with neovascular AMD. Paper presented at American Society of Retina Specialists 2020 virtual annual meeting; July 26, 2020.



