 |
Recent reports have suggested that retinal diabetic neuropathy (RDN) is an independent process, developing and progressing irrespective of the presence or severity of diabetic microvasculopathy. RDN manifests on optical coherence tomography as significant thinning of the retinal nerve fiber layer (NFL) and ganglion cell and inner plexiform layers (GCL+IPL).3
Many authors, including ourselves, have shown that this degeneration occurs in people with diabetes regardless of clinical markers of metabolic control. Progressive thinning can eventually lead to visually significant changes, such as loss of contrast sensitivity, dark adaptation and peripheral field loss.2 Recognizing these changes is important for patient management and can lead to better understanding of the disease process, as well as novel therapies.
Diabetic Retinopathy Burden
In 2014, at least 422 million people were estimated to have diabetes mellitus worldwide, which is almost as many individuals affected during the “Spanish” influenza pandemic of 1918.4
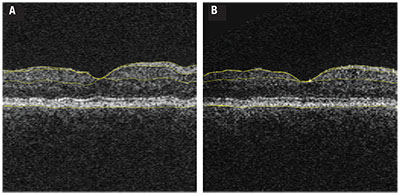 |
| Figure 1. Optical coherence tomography with segmentation performed in a fashion similar to that used in the longitudinal study analysis1 in a patient with diabetes at the outset of the longitudinal analysis (A) compared to the same patient at the final follow-up demonstrating the cumulative thinning over the study duration (B). Yellow automated segmentation outlines the internal limiting membrane, nerve fiber layer-ganglion cell layer boundary, inner plexiform-to-inner nuclear layer boundary and basement membrane. |
Beyond the massive economic impact, DR accounts for a significant component of diabetes-related morbidity. It is the leading cause of vision loss in people with diabetes, and it is one of the chief causes of irreversible blindness in working-age adults across the world.7
Microvascular Damage Not the Whole Story
Traditional thinking has held that diabetes affects the eye through microvascular endothelial damage caused by advanced glycosylated end products. Histological analyses have shown that the earliest notable microvascular changes are pericyte loss followed by acellular capillaries.8,9 This diabetic microvasculopathy manifests funduscopically with the well-known features of microaneurysms, venous beading, lipoprotein exudates and retinal edema.10
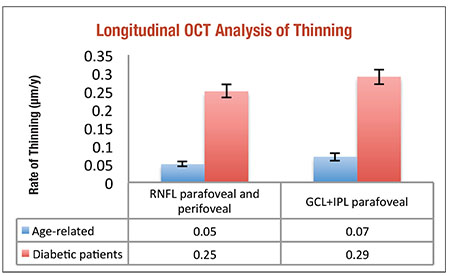 |
| Figure 2. Progressive neuroretinal thinning (per year) in people with diabetes1 far surpasses age-related thinning in people without diabetes.25 |
Also, HbA1c is well established as an accurate marker for diabetic control and microvascular damage. However, the Epidemiology of Diabetes Interventions and Complications/Diabetes Control and Complications Trial studies have shown that HbA1c explains only 11 percent of the variance in developing or worsening DR, indicating other factors are involved.12 Recent evidence and investigation suggests that microvascular damage is not the complete story regarding diabetic ocular disease.
RDN as an Independent Entity
Histological analysis of postmortem human retinal tissue in people with diabetes identified retinal neurodegenerative changes such as loss of ganglion cell bodies, glial reactivity and neural apoptosis.13 These changes are evident in vivo as thinning of the neuroretinal layers, which are measurable on OCT through image analysis.14
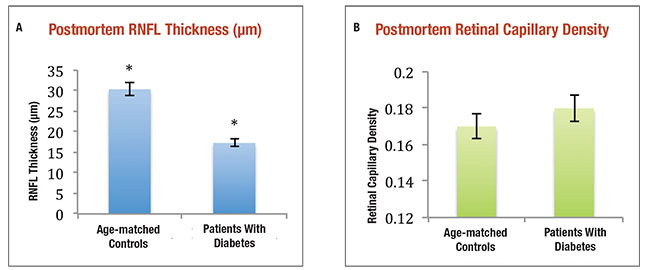 |
| Figure 3. The average retinal nerve fiber layer thickness of cadaveric human eyes with diabetes but no or minimal diabetic retinopathy was significantly thinner than controls (A) (* indicates p-value <0.05), but retinal capillary densities were not significantly different between the same human donor subjects (B).1 |
Recently, we showed with our collaborators that the NFL, GCL and inner plexiform layer (IPL) were thinner in people with diabetes when compared to age-matched controls.2 These subjects had no or minimal DR on funduscopic exam.
Other studies in patients with diabetes and animal models have confirmed these findings, and a commonality among them is that retinal neurodegeneration, quantified either structurally or functionally, occurs when the retina also manifests microvasculopathy from diabetes.2,15-17 However, the precise temporal relationship between microvasculopathy and RDN was not known until we studied this more carefully. We published these results earlier this year.1
Investigation into the pathophysiology of RDN has shown mechanisms such as oxidative stress, extracellular glutamate accumulation and a relative loss of neuroprotective factors synthesized by the retina
to be likely causal of degeneration, and possibly independent of vasculopathy.18,19
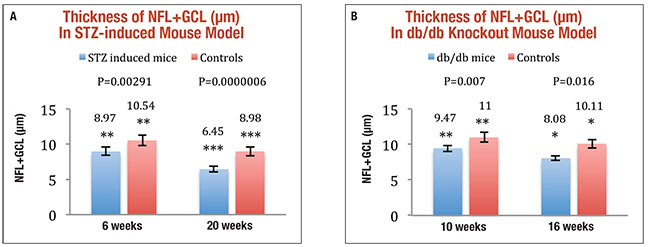 |
| Figure 4. Streptozotocin-induced diabetic mice had significantly thinner nerve fiber layer and ganglion cell layer (NFL+GCL) than age-matched controls at both six weeks and 20 weeks after induction (A). Comparison of NFL+GCL thicknesses in db/db knockout mice (spontaneously diabetic) against age-matched controls at ages 10 and 16 weeks showed a less-significant difference (B).1 |
Some investigations failed to find an association between RDN and other traditional markers of diabetes severity, such as HbA1c.2 Although HbA1c correlates highly with proliferative DR, it may not be associated with RDN.20 Others have also suggested that neurodegeneration is secondary to diabetes, but not related to diabetic vasculopathy, based on systemic investigations. One example is that some studies show that people with diabetes can develop brain atrophy over time without an increase in the number of infarctions.21 RDN has also been shown to be directly related to peripheral neuropathy in severity.22
Clinical Significance of RDN
In glaucoma, progressive neuroretinal thinning is the likely mechanism of peripheral and eventual central vision loss. Thus, it is not unreasonable to think that RDN has a similar effect on vision. Our own reports have shown an association between the severity of RDN and vision loss on perimetry.2,15 Others have also reported functional deficits of RDN via measurable abnormalities in electroretinography (ERG) and loss of contrast sensitivity and dark adaptation.17
Anthony Adams, OD, PhD, and colleagues recently studied ERGs in subjects with diabetes but no baseline DR and found that early ERG changes can predict if and where future DR, such as microaneurysms, will develop.16 Investigations in both animal and human models have strongly supported the idea that RDN is a separate disease process from diabetic microvasculopathy, but none have established which came first.
The need to better determine whether RDN or diabetic microvasculopathy occurs first is clear, because this can have profound effects on how clinicians manage people with diabetes and what the focus of research on prevention of vision loss should be. The results could also impact the relationship of diabetes-related eye changes to other neural changes in the peripheral and central nervous systems.
Based on the classic World Health Organization (WHO) screening criteria applicable to all conditions, RDN would not currently qualify.23 Currently, WHO recommendations for detecting or screening for diabetic retinopathy include various modalities such as photography and dilated fundus examination.24 Although no known treatment or way to prevent RDN exists, expansion of these protocols to include evaluation of RDN may be warranted in the future.
Human Studies of RDN
To better understand the relative timing of whether RDN or microvasculopathy occurs first, we evaluated retinal changes in humans with diabetes and in two different mouse models.1 We studied human eyes via a prospective OCT analysis study as well as histological analysis of donor eyes of people with diabetes compared to controls. In both of these studies, the subjects with diabetes had no DR or minimal signs.
Our prospective study involved a cohort of 45 people with diabetes with no or minimal DR, following them with annual OCT analysis over an average of 73 months. Subjects had a mean HbA1c of 8.2 percent at baseline. We performed regular measurements of HbA1c, color fundus photography and OCT imaging. Subjects developed significant inner retinal thinning over the course of the study regardless of their DR status (Figure 1, page 33).
NFL thinning occurred at an average rate 0.25 μm/year; parafoveal GCL+IPL thinning occurred at a rate of 0.29 μm/year, after correction for age, sex, HbA1C status, DR status, blood pressure, DR progression and duration of diabetes. This thinning was statistically significant when compared to the normal age-related rate of thinning, and was readily apparent over the duration of the study (Figure 2).
Using the same methods, our collaborators previously established the normal rate of age-related thinning of the NFL and GCL at 0.05 μm/year and 0.07 μm/year respectively.25 We found that neuroretinal thinning correlated with the duration of diabetes before inclusion, but we found no correlation between HbA1c level and development of RDN.
In donor eyes of people with diabetes but no or minimal DR, we compared neuroretinal thickness to similarly aged controls without diabetes. The NFL was found to be significantly thinner in the DM eyes, 17.3 μm, compared to controls, 30.4 μm (p=0.03) (Figure 3).1 We noted no statistically significant difference in retinal capillary density between the two groups, indicating a lack of microvascular damage. Thus, there is RDN in human donor eyes of people with diabetes that do not have clinical manifestation of DR or detectable microvascular changes on histological analysis.
Timing of RDN and Diabetic Microvasculopathy
In this same study, we evaluated RDN in two mouse models,1 the findings of which corroborated the human data. A streptozotocin-induced mouse model of type 1 DM was used to show an average retinal thinning of 17.5 percent and 39.2 percent at six weeks and 20 weeks, respectively, using OCT analysis when compared to age-matched controls. Ganglion-cell density decreased as well, though there was no presence of diabetic microvasculopathy via histological analysis (loss of pericytes or acellular capillaries) at any time point in either group.1
Because some authors have suggested that streptozotocin itself could cause neural damage, we looked at a second model of mice that had spontaneous diabetes mellitus due to genetic mutation. In this db/db knockout mouse model that simulates type 2 diabetes, we compared retinal thickness against age-matched controls via OCT. The db/db mice were again found to have significantly thinner NFL+GCL by 13.9 percent and 20.1 percent at 10 weeks and 16 weeks of age, respectively (Figure 4, page 35).
These findings support the notion that RDN occurs before, and is independent of, diabetic microvasculopathy. For a more detailed description of our methods and results, please see the article we co-authored earlier this year in the Proceedings of the National Academy of Sciences of the United States of America.1
Future Trends in Management
If duration of diabetes could be found to correlate with clinically meaningful loss of function after a certain period of time, further studies could be useful in establishing monitoring guidelines and recommendations. Since ERG has been shown to predict development and location of DR, it could prove useful for early risk stratification and follow-up evaluation of people with diabetes with no baseline DR on exam.
Knowing who will develop microvasculopathy, and specifically when, is potentially useful for patient management. It may also be informative to compare perimetric analysis of patients with diabetes and those with glaucoma and similar amounts of NFL and GCL loss to determine if similar deviations exist. A study from our institution has shown a difference of 5 to 8 μm of the NFL and 1 to 8 μm of the GCL in patients with early vs. severe stages of glaucoma, and that these changes impart meaningful perimetric deficits.26
Based on data presented previously, diabetes patients could reach a level of retinal thinning near severe glaucoma over the course of 10 to 20 years. Because perimetry is used routinely to track glaucomatous progression, it may have a role in the assessment of DR, specifically longstanding RDN.
Currently we have no known agents to stop the development of RDN, though some candidates exist.27 Now that we know that RDN precedes microvasculopathy, any neuroprotective agent that slows the progression of RDN may prove useful in concurrently slowing the development of microvasculopathy and severe vision-threatening DR if such a causal relationship exists.
The use of OCT and OCT angiography for early detection could provide an opportunity for a timely and economically feasible study to investigate preventative therapies early in the disease.
A Paradigm Shift
These findings present a significant evolution in our understanding of the pathophysiology of how diabetes affects the retina, and has implications for all diabetes-related complications. Much current literature supports systemic diabetic neuropathy as an independent ischemic process. Our published data supports the thought that RDN is not ischemic in origin, but is instead an antecedent process that has the potential to be a causal factor in DR and microvasculopathy in the retina and elsewhere.
| Take-home Point Emerging reports have supported the idea that retinal diabetic neuropathy (RDN) is an independent ischemic process. This article explores the authors’ own study that found inner retinal degeneration in eyes that had no funduscopically evident diabetic retinopathy, and explores data that RDN is not ischemic in origin, but is instead an antecedent process that may be a causal factor in diabetic retinopathy and microvasculopathy in the retina and elsewhere. |
As the study of diabetic neuropathy continues, retina specialists are afforded a unique opportunity for analysis, given our current modes of visualizing retinal disorders. As our knowledge develops, it may become important to use spectral domain OCT analysis to quantify RDN regardless of the presence of DR in people with diabetes. This knowledge could alter practice patterns and provide a turning point in the management and understanding of the disease. RS
REFERENCES
1. Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci USA. 2016;113:E2655-2654.
2. van Dijk HW, Verbraak FD, Kok PH, et al. Early neuro-degeneration in the retina of people with type 2 diabetes. Invest Opthalmol Vis Sci. 2012; 53:2715-2719.
3. Jeon SJ, Kwon JW, La TY, Pak CK, Choi JA. Characteristics of retinal nerve fiber layer defect in nonglaucomatous eyes with type 2 diabetes. Invest Ophthalmol Vis Sci. 2016; 57:4008-4015.
4. Yates T, Khunti K. Epidemiology: The diabetes mellitus tsunami: worse than the ‘Spanish flu’ pandemic? Nat Rev Endocrinol. 2016; 12:377-378.
5. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013; 36:1033-1046.
6. Summers KHR, Ryan GJ. The economic impact of diabetic retinopathy and the promise of emerging therapies [CE Activity]. International Medical Press; 2007.
7. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years), 1999-2000 with 2009-2010. BMJ Open. 2014; 4:e004015.
8. Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961; 66:366-378.
9. Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996; 114:986-990.
10. Friedenwald JS. Diabetic retinopathy. Am J Ophthalmol. 1950; 33:1187-1199.
11. American Academy of Ophthalmology. 2015-2016 Basic and Clinical Science Course: Retina and Vitreous. San Francisco, CA: American Academy of Ophthalmology.
12. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. 2008;57:995-1001.
13. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiology. 2008; 586:4401-4408.
14. Abramoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE Rev Biomed Engin. 2010; 3:169-208.
15. van Dijk HW Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011; 51:224-228.
16. Adams AJ, Bearse MA Jr. Retinal neuropathy precedes vasculopathy in diabetes: a function-based opportunity for early treatment intervention? Clin Experiment Optom. 2012; 95:256-265.
17. Bronson-Castain KW, Bearse NA Hrm Beyvukke Hm et al. Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina. 2009; 29:618-626.
18. Simo R, Hernandez C, European Consortium for the Early Treatment of Diabetic Retinopathy. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014; 25:23-33.
19. Stem MS, Gardner TW. Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications. Curr Med Chem. 2013; 20:3241-3250.
20. No authors listed. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995; 44:968-983.
21. van Elderen SG et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 2010; 75:997-1002.
22. Srinivasan S, Pritchard N, Vagenas D, et al. Retinal tissue thickness is reduced in diabetic peripheral neuropathy. Curr Eye Res. 2016:1-8.
23. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanita Panam. 1968;65:281-393.
24. Prevention of Blindness from Diabetes Mellitus. World Health Organization ed. Report of a WHO Consultation; November 9-11, 2005; Geneva, Switzerland. http://www.who.int/entity/blindness/Prevention%20of%20Blindness%20from%20Diabetes%20Mellitus-with-cover-small.pdf?ua=1 Accessed October 7, 2016.
25. Ooto S, Hangai M, Tomidokoro A et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci. 2011; 52:8769-8779.
26. Bogunovic H, Kwon YH, Rashid A, et al. Relationships of retinal structure and humphrey 24-2 visual field thresholds in patients with glaucoma. Invest Ophthalmol Vis Sci. 2015; 56:259-271.
27. Trammell SA, Weidemann BJ, Chadda A, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016; 6:26933.



