 |
 |
 |
 |
The coronavirus pandemic may have forced the cancellation of this year’s meeting of the Association for Research in Vision and Ophthalmology, but the call was made late after all those ARVO abstracts had been submitted, reviewed and accepted —and published online.
Here, we present our annual selection of five compelling posters and presentations. We begin with three promising therapies designed to significantly reduce the treatment burden for neovascular age-related macular degeneration. The last two abstracts discuss sildenafil as a potential treatment for central serous chorioretinopathy (CSC) and the utility of internal limiting membrane peeling for repair of rhegmatogenous retinal detachments.
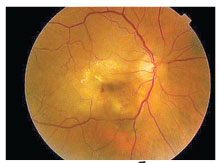 |
First in-human results of single-injection sunitinib
Given the rapidly increasing aging population, the need for increased durability to reduce treatment burden for nAMD has become paramount. Sunitinib, a pan-
vascular endothelial growth factor receptor inhibitor, has been formulated as an intravitreal depot (GB-102, Graybug Vision) designed to increase treatment intervals to every six months or more.
ADAGIO is a multicenter, open-label, escalating dose-cohort Phase I/IIa study of 32 patients who’ve had nAMD for less than 18 months.1 All patients received at least three prior anti-VEGF injections and demonstrated response to treatment. Intravitreal GB-102 was administered at a dose of 0.25, 0.5, 1 or 2 mg, and the patients were then followed monthly for eight months. If certain criteria were met, they received supportive aflibercept (Eylea, Regeneron Pharmaceuticals) therapy.
At baseline, mean best-corrected visual acuity was 63.3 letters and mean central subfield thickness 294 μm. Patients received an average of 4.8 anti-VEGF treatments before enrollment.
The presence of transient medication residue was the most frequently reported adverse event (n=9). The proportion of patients that did not require additional supportive anti-VEGF treatment at three, six and eight months was 88, 68 and 42 percent, respectively. No dose-limiting toxicities, ocular serious adverse events or infections were reported.
Existing anti-VEGF treatments permit treatment intervals of up to three months in certain patients. GB-102 maintained an acceptable level of disease activity in a cohort of nAMD patients for up to eight months without additional treatment. GB-102 demonstrated potential to reduce treatment burden in nAMD, but further study is warranted.
Disclosures: Lead author Charles Semba is an employee of Graybug Vision, and four coauthors disclosed financial relationships with Graybug.
 |
IVT gene therapy suppresses disease activity to 11 months
 |
ADVM-022 (AAV.7m8 aflibercept gene therapy, Adverum Biotechnologies) is an intravitreal gene therapy designed to provide sustained therapeutic levels of aflibercept after a single injection. The
OPTIC Trial is an open-label, multicenter, dose-ranging study in previously treated nAMD patients with confirmed response to anti-VEGF therapy.2 A single intravitreal injection of ADVM-022 was administered at 6x1011 vg/eye in cohort 1 (n=6) and 2x1011 vg/eye in cohort 2 (n=6). Patients meeting certain criteria would receive rescue aflibercept therapy.
Cohort 1 patients required a mean of 6.2 anti-VEGF injections in the eight months prior to enrollment to maintain their baseline mean BCVA of 65.8 letters. Any postinjection inflammation was generally
anterior, mild and manageable with topical steroids. At a median of 34 weeks of follow- up (range: 24 to 44 weeks), no patients required rescue injections. No dose-limiting toxicities, serious ocular adverse events or endophthalmitis were reported.
ADVM-022 maintained an acceptable level of disease activity in a cohort of nAMD patients for up to 11 months without additional treatment.2
Disclosures: Lead author Arshad M. Khanani, MD, and co-authors disclosed financial relationships with Adverum Biotechnologies, three of whom are employees.
 |
Anti-VEGF biopolymer conjugate extends treatment
The final potential therapeutic agent with increased durability is KSI-301 (Kodiak Sciences), a novel anti-VEGF antibody biopolymer conjugate. In a Phase Ib study, treatment-naïve patients with nAMD, diabetic macular edema or retinal vein occlusion received three initial monthly doses of either 2.5 mg or 5 mg KSI-301.3 Additional treatment for each disease was based upon protocol-specified retreatment criteria.
Among 55 patients, mean BCVA change at week 20 was +4.3 letters in nAMD (n=25, baseline 64.5), +7.4 letters in DME (n=15, baseline 66.8) and +21.3 letters in RVO (n=15, baseline 54.9). Mean change in CST was -67 µm in nAMD (baseline 426), -129 µm in DME (baseline 449) and -365 µm in RVO (baseline 675).
Treatment was extended to three months or longer in 92 percent of nAMD eyes after the last loading dose without retreatment; 72 percent of DME eyes were extended to four months or longer; and half of RVO eyes were extended to three months or longer. In a total of 338 doses, no reports of intraocular inflammation and no drug-
related adverse events were noted.
KSI-301 demonstrated excellent efficacy and durability and had no reported cases of intraocular inflammation in this study. If the vision outcomes and safety profile are similar in larger patient cohorts, this could prove to be a very intriguing treatment for multiple common retinal diseases.
Disclosures: Lead author Sunil S. Patel, MD, is a consultant to Kodiak Sciences, and all coauthors are employees of Kodiak Sciences.
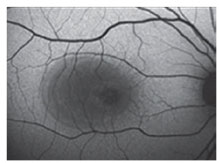 |
CSC shows treatment response to sildenafil
Oral sildenafil, best known as the erectile dysfunction drug Viagra, is a systemic inhibitor of phosphodiesterase 5 and 6 (PDE5/6). In patients with CSC, it may reduce subretinal fluid and improve vision. Inhibition of PDE5/6 enhances the effects of nitrous oxide, which leads to increased choroidal blood flow. This may accelerate the resorption of SRF in CSC.
Four patients with refractory CSC were treated with oral sildenafil 20 or 40 mg b.i.d. and followed for an average of four months.4 All patients demonstrated resolution of SRF. Mean time to resolution of SRF was two to three months. Mean improvement in visual acuity was 7 letters.
The authors report that sildenafil was well-tolerated by all patients. Larger studies are warranted, but this may be a promising new agent for the treatment of chronic and/or recurrent CSC.
Disclosures: The study authors have no relationships to report.
 |
ILM peeling during RRD surgery
Recent publications have led retina specialists to debate the need for concurrent peeling of the ILM during pars plana vitrectomy for RRD. Some believe that ILM peeling can reduce the rate of postoperative epiretinal membrane and proliferative vitreoretinopathy formation. Matthew Starr, MD, and colleagues performed a multi-institutional retrospective review of all RRD surgeries involving PPV for a full calendar year (2015) and compared the outcomes between eyes with and without ILM peeling.5
The series included 1,287 eyes, 87 (6.8 percent) of which underwent ILM peeling at the time of surgery. Final mean visual acuity was significantly better in the group without ILM peeling (logMAR 0.44 ±0.57, Snellen 20/55) vs. eyes with ILM peeling (logMAR 0.64 ±0.66, Snellen 20/87, p=0.0096).
A multivariate analysis that accounted for four preoperative factors—VA, PVR, and macular hole and macular attachment status—found no difference in postoperative
visual acuity between eyes with and without ILM peeling (p=0.2373).
The single-surgery success rate was not significantly different between the two groups: 86.2 percent with ILM peeling vs. 83 percent without (p=0.5571). The rate of postoperative ERM formation was also not significantly different: 29.1 percent vs. 35.7 percent, respectively, (p=0.1330).
The debate continues regarding the utility of ILM peeling in RRDs. This large retrospective review should give us pause when deciding to peel the ILM in PPVs for RRDs, but we need more robust prospective data. RS
Disclosures: The study authors have no relationships to report.
REFERENCES
1. Semba C, Patel SS, Kunimoto DK, et al. A novel intravitreal depot formulation of sunitinib (gb-102): Eight-month results of a Phase I/IIa first-in-human single injection study in patients with neovascular age-related macular degeneration (ADAGIO Study). Association for Research in Vision and Ophthalmology 2020 abstract #4918-B0123.
2. Khanani AM, Kiss S, Turpcur A, Hoang C, Osborne A. Phase 1 Study of Intravitreal Gene Therapy ADVM-022 for Neovascular AMD (OPTIC Trial). ARVO 2020 abstract #1154.
3. Patel SS, Janer D, Miller B, Perloth V, Valezquez-Martin JP. Updated Results of Phase 1b Study of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate with Extended Durability, in wAMD, DME, and RVO. ARVO 2020 abstract #4286.
4. Daly S, Coleman DJ, Breazzano M, et al. Central serous chorioretinopathy treatment with a systemic PDE5/6 inhibitor (sildenafil. ARVO 2020 abstract # 1071.
5. Starr M, Patel L, Ammar MJ, et al. Utility of internal limiting membrane peeling during rhegmatogenous retinal detachment surgery. ARVO 2020 Abstract #3527.



