Take-home Points
|
 |
|
Bio Dr. Abbey is director of clinical research at Texas Retina Associates, Dallas, and a clinical assistant professor of ophthalmology at the University of Texas DISCLOSURES: Dr. Abbey is a consultant to Alcon, Allergan/AbbVie, Alimera Sciences, EyePoint |
The 39th annual scientific meeting of the American Society of Retina Specialists returned to a live format last month, albeit with a virtual component, after last year’s all-virtual meeting. Despite the challenges of travel during the pandemic, retina specialists from around the world managed to converge on San Antonio.
Here, we present five notable abstracts from the meeting: a post-hoc analysis of DRCR Research Network Protocols T and V; results of the INFINITY Phase II trial of the one-time gene therapy ADVM-022 in diabetic macular edema; early results from the first cohort enrolled in the ALTITUDE trial of another one-time gene treatment, RGX-314, also in DME; a comparison of two treatment regimens for vitreous hemorrhage in proliferative diabetic retinopathy; and a retrospective case series of anti-VEGF treatment failures in infants with retinopathy of prematurity.
 |
Fluctuations in central subfield thickness in DME
A post-hoc analysis used databases from the DRCR Research Network’s Protocols T and V to explore the question if large fluctuations in central subfield thickness in patients with diabetic macular edema lead to worse vision over time.1 The answer is, they may.
The study included 1,197 eyes, 559 from Protocol T and 638 from Protocol V. All eyes had at least three CST readings and visual acuity recordings at one year. The primary outcomes were VA at one and two years for each protocol, with the patients grouped into quartiles, presenter Matthew Starr, MD, said.
The study found significant VA differences based on the standard deviation of CST quartiles for both protocols while adjusting for mean baseline VA, baseline CST, lens status, hemoglobin A1c and treatment arm. The first quartile had the least fluctuation and served as the reference group. The fourth quartile had the greatest fluctuation.
In Protocol T, the difference between the first and fourth quartiles after one year was -1.61 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (95% CI, -3.51 to 0.30, p=0.986) and -3.59 (95% CI, -6.17 to -1.00, p=0.0066) after two years.
In Protocol V at one year, the difference between the first and fourth quartiles was -3.04 ETDRS letters (95% CI, -4.18 to -1.91, p<0.0001). At two years, the difference was -2.35 letters (95% CI, -3.58 to -1.13, p=0.0005).
In Protocol V, a higher proportion of eyes in the first quartile received anti-VEGF treatment than in the fourth quartile, raising the question of whether treatment frequency needs to be increased or if dual therapy with corticosteroids should be considered.
Dr. Starr said the primary study takeaways are that greater fluctuations in macular edema appear to be associated with worse vision outcomes in DME patients; that this method of analyzing CST may provide better insight into VA outcomes; and that CST fluctuations and the role of therapeutic agents and/or treatment intervals that limit CST fluctuations warrant further study.
Dr. Starr, of Mayo Clinic, Rochester, Minn., has no relevant disclosures.
 |
Intravitreal gene therapy for DME: Results of Phase II Infinity trial
Adverum Biotechnologies sponsored the Phase IIINFINITY trial to evaluate ADVM-022,2 a single-injection intravitreal adeno-associated virus 7m8 gene therapy designed to create an intraocular aflibercept biofactory to reduce treatment burden in DME. While Adverum had already decided to discontinue the DME program for ADVM-022, the company still presented trial results.
The trial enrolled 36 patients with newly diagnosed DME–that is, within six months of screening–and who had received up to two previous anti-VEGF injections in the study eye. The patients received either a standard-of-care bolus of aflibercept or a single intravitreal injection of ADVM-022 in one of two doses: low dose (2 x 1011 vg/eye) or high dose (6 x 1011 vg/eye). They were evaluated monthly for 48 weeks.
Presenter Charles C. Wykoff, MD, PhD, said the study was unmasked in May after a patient who had severe comorbidities in the higher-dose ADVM-022 group developed hypotony. Two additional cases that required surgery were reported later. The inflammation in the higher-dose patients may have been related to the comorbid nature of the study population. Both ADVM-022 arms had higher rates of serious ocular adverse events than the aflibercept arm.
 |
All three treatment arms showed improvements in visual acuity, but after week 24 the high-dose ADVM-002 group had a drop-off that continued through week 34. CST improvements were observed in all three arms with no meaningful differences. At weeks 12 and 24, more patients in the gene therapy arms also showed two- and three-step improvement in Diabetic Retinopathy Severity Scale compared to the aflibercept arm.
The rates of nonocular adverse events were similar between the arms, but 20 of 25 patients in the ADVM-022 arms developed anterior intraocular inflammation, three developed posterior IOI, and 15 had an iris-related event.
Notably, the OPTIC trial of ADVM-022 in patients with neovascular age-related macular degeneration demonstrated an acceptable safety profile while reducing the need for anti-VEGF injections by more than 80 percent while stabilizing central subfield thickness. Evaluating the differences in safety profiles between the two studies is a focus of ongoing research. Meanwhile, while Adverum has discontinued the program in DME, it’s still investigating the lower dose in nAMD.
Dr. Wykoff, is a partner in Retina Consultants of Texas and deputy chair of ophthalmology at Blanton Eye Institute, Houston. He is a member of Adverum Biotechnologies’ scientific advisory board, and serves as an investigator for and receives grants from the company.
 |
Early first-cohort results of suprachoroidal gene therapy for CI-DME
The Phase II ALTITUDE study is evaluating another single-administration gene therapy, RGX-314 (RegenxBio) in patients who have diabetic retinopathy without center-involved DME. RGX-314 is administered into the suprachoroidal space and uses the NAV AAV8 vector to deliver a soluble anti-VEGF fab transgene to provide continuous anti-VEGF expression.
Dennis M. Marcus, MD, reported on 20 eyes that have been enrolled in the first cohort of the study.3 At three months, the rate of DRSS improvement in the RGX-314 group (n=15) was 33 percent vs. zero percent in the observation group (n=5). In the observational group, three patients had no change in DRSS, one patient had a one-step improvement and one had a two-step worsening. Among the RGX-314 patients, four had no change, three had a one-step improvement, four a two-step improvement and one a four-step gain. Three RGX-314 patients had a worsening of DRSS, two by one step, and one by two steps.
The treatment was well-tolerated, Dr. Marcus said. One vitreous hemorrhage occurred in a fellow eye. Common adverse events included conjunctival hyperemia and hemorrhage, which were predominantly mild and weren’t considered to be drug-related. One case of mild episcleritis, reported two weeks after suprachoroidal administration of RGX-314, resolved with topical corticosteroids.
 |
The overall early results of ALTITUDE fall within the range of three-month results of DME trials of aflibercept and ranibizumab, Dr. Marcus said. In a subgroup of RGX-314-treated patients with DR levels of 47 to 53, 43 percent had a two-step improvement in DRSS.
ALTITUDE is enrolling Cohorts 2 and 3 using a dose level of 5 x 1011 GC/eye with NAb- and NAb+ patients.
Dr. Marcus is a vitreoretinal surgeon at Southeast Retina Center in Augusta, Georgia. He is a consultant to and receives research grants from RegenxBio.
Aflibercept vs. vitrectomy/PRP for PDR hemorrhage
A vitreous hemorrhage from proliferative diabetic retinopathy can cause acute vision loss. Hani Salehi-Had, MD, reported results of DRCR Retina Network Protocol AB that compared two treatment plans: vitrectomy with panretinal photocoagulation, with aflibercept pro re nata (n=105); or 2-mg aflibercept with vitrectomy PRN (n=100).4 Ninety-six percent of eyes in the aflibercept group and 85 percent in the vitrectomy group completed the two-year study.
The vitrectomy/PRP group had surgery within two weeks of randomization and, in the event of recurrent vitreous hemorrhage, received two monthly aflibercept injections and additional injections every four weeks as needed. The aflibercept group received injections at baseline and every four weeks up until week 12, with an evaluation at week 16 to defer injections if the therapy succeeded or for vitrectomy if the VH persisted. All participants had visits at weeks four and 12, then every 12 weeks out to two years.
Dr. Salehi-Had noted that mean visual acuity at 24 weeks was similar between the two groups, slightly favoring the vitrectomy group by 5 letters (p=0.06). Mean visual acuity at four weeks was 20/100 in aflibercept group and 20/63 in the vitrectomy/PRP group (p=0.003).
Mean visual acuity at 24 weeks and two years was 20/40 in each group. At 24 weeks, 70 percent of the aflibercept patients and 72 percent of the vitrectomy/PRP patients had >15-letter gains (p=0.99). At two years, those percentages were around 75 percent. On the loss side, 5 and 11 percent of patients lost >15 letters. Fewer patients in the aflibercept group had DME at 24 weeks, but at two years the difference was negligible.
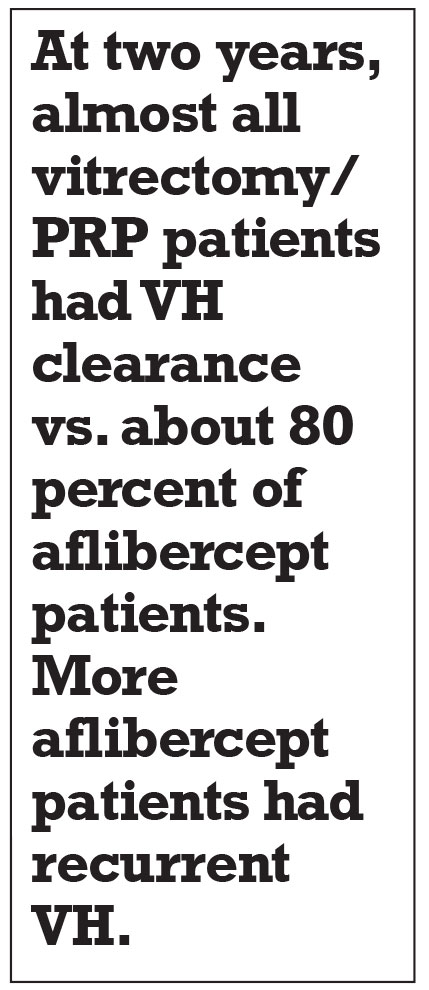
At two years, almost all vitrectomy/PRP patients had VH clearance vs. about 80 percent of the aflibercept patients (p=0.001). More aflibercept patients had recurrent VH. About one-third of patients in each group received the alternative treatment.
Dr. Salehi-Had, a vitreoretinal surgeon at Retina Associates of Southern California in Orange County, has no relevant disclosures.
 |
Infants with ROP who fail anti-VEGF therapy
A retrospective case series sought to identify and describe characteristics of infants with retinopathy of prematurity who fail anti-VEGF therapy.5 Lucy T. Xu, MD, presented results of 211 eyes of 112 babies treated with anti-VEGF as initial therapy for type 1 ROP from 2011 to 2019 at Children’s Healthcare of Atlanta. The study received the ASRS Fellows Forum Award.
The study population included six eyes of three patients who had been referred to the institution after they failed anti-VEGF treatment at outside centers. The study analyzed 23 eyes of 15 patients who failed treatment and 194 eyes of 100 patients in whom treatment succeeded.
The study used four separate criteria for treatment failure: need for repeat anti-VEGF treatment or laser before post-menstrual age (PMA) of 50 weeks; recurrent-plus; recurrent Stage 3; or Stage 4 or 5 ROP at any PMA.
Twenty-two of the failed eyes received bevacizumab; one was treated with ranibizumab outside the institution. The study found no association between treatment failure and bevacizumab dose, and the median time to failure was 10.1 weeks. Three eyes had first failure after 50 weeks PMA.
The study also elucidated five different manifestations of initial treatment failure: recurrent stage 3 (n=13, 56.5 percent); recurrent plus (n=11, 47.8 percent);
 |
retinal detachment (n=5, 21.7 percent); vascular arrest in zone I (n=2, 8.7 percent); and vitreous hemorrhage (n=1, 4.3 percent).
In all, nine of the failed eyes had RDs, including the five that had them with initial treatment failure. Of the remainder, three had RD with the second failure, all ow which were previously treated with laser; and one with the third failure after previous combined intravitreal bevacizumab/laser treatment followed by laser treatment alone.
The median follow-up was two years. Eighteen eyes, including all RD eyes, had follow-up of six months or more. The retina was fully attached in all but one eye, and fixation behavior was present in 10 eyes–but in only two of nine eyes that had RD.
Dr. Xu noted that the most common manifestations of treatment failure were recurrent plus and recurrent Stage 3. Almost all of the eyes that failed anti-VEGF treatment ultimately had favorable anatomic outcomes and half demonstrated fixation behavior, she said.
Dr. Xu, a vitreoretinal surgeon with The Retina Group of Washington in the Washington, D.C., region, has no relevant disclosures. Study authors received funding from the National Eye Institute. RS
REFERENCES
1. Starr M, Salabati M, Mahmoudzadeh R, et al. Fluctuations in central subfield thickness associated with worse visual outcomes in patients with diabetic macular edema in clinical trial setting. Paper presented at American Society of Retina Specialists 39th annual scientific meeting. San Antonio, TX; October 9, 2021.
2. Wykoff CC, Emanueli A, Barakat MR, et al. Intravitreal gene therapy for diabetic macular edema with ADVM-022: First-time data presentation of prospective, randomized Phase 2 INFINITY Trial. Paper presented at ASRS. San Antonio, TX; October 9, 2021.
3. Marcus DM. Suprachoroidal Delivery of RGX-314 for diabetic retinopathy without CI-DME: Early results from the Phase II ALTITUDE study. Paper presented at ASRS. San Antonio, TX; October 9, 2021.
4. Salahi-Had H. Aflibercept versus vitrectomy with panretinal photocoagulation for vitreous hemorrhage from proliferative diabetic retinopathy. Paper presented at ASRS. San Antonio, TX; October 9, 2020.
5. Xu LT, Levine D, Hutchinson A, Rao P, Hubbard GB. Clinical features and outcomes of infants with retinopathy of prematurity who fail Anti-VEGF therapy. Paper presented at ASRS. San Antonio, TX; October 9, 2021.



