 |
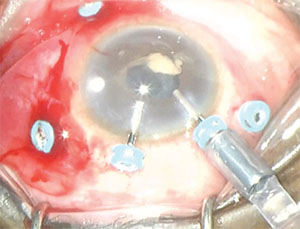
| Figure 1. Early vitrectomy for postoperative infectious endophthalmitis involves a modification of the standard three-port posterior vitrectomy technique with two additional trocar/cannulas at the corneal limbus. The online video available at http://bit.ly/2aWlzk7 describes the technique in its entirety. |
Interestingly, the EVS findings showed that 26 percent of patients had no pain on presentation and 14 percent did not have a hypopyon.1 Additionally, 94.2 percent of cultures confirmed gram-positive bacteria, mostly coagulase-negative Staphylococcus epidermidis.1
Thanks to MIVS, today we feel that early vitrectomy for endophthalmitis may be of significant benefit because it removes the infectious material and vitreous debris that are paramount to accelerating the clearance of the infection and optimizing visual outcomes, respectively.
Where EVS relied on older vitrectomy techniques with known increased rates and severity of complications, such as retinal detachment and vitreous hemorrhage, MIVS in contrast provides a quicker and safer option for eyes with severe inflammation. We recently reported 10-year data that showed small-gauge vitrectomy for endophthalmitis yields final visual outcomes comparable to 20-gauge instrumentation.2 In vitro laboratory testing revealed no significant difference in rates of culture growth for different vitrectomy gauge sizes or vitreous cutting speeds.3
Our Approach For Endophthalmitis
Our preferred technique for infectious endogenous endophthalmitis is vitreous biopsy (“tap”) via a short 25-gauge needle on a 3- or 5-mL syringe. This is followed by injection with intravitreal antibiotics at the pars plana in the clinic. The most common antibiotics we use are intravitreal ceftazidime 2.25 mg/0.1 mL and vancomycin 1 mg/0.1 mL. In cases of known serious penicillin allergy, intravitreal amikacin 400 mcg/0.1 mL could be considered.
Additionally, we often use intravitreal dexamethasone 400 mcg/0.1 mL as an adjunct to address the severe secondary inflammation when our suspicion for fungal etiologies is low. If we cannot obtain a vitreous sample because the vitreous fluid is too viscous, we obtain an aqueous sample for cultures instead via a short 30-gauge needle on a 1-mL syringe at the limbus. (Note: the aqueous samples in the EVS were positive in only 42 percent of eyes.1)
The clinical presentation can sometimes worsen within 24 hours of antibiotic injection. If the patient shows no clinical improvement, we typically perform pars plana vitrectomy within 48 to 72 hours of initial presentation, with the idea that the vitreous acts as a culture medium for microorganisms. This is speculated to be the most likely cause of the low incidence of endophthalmitis following routine pars plana vitrectomy surgery.
Five-trocar Setup
When the endophthalmitis does not resolve after our surgical technique described here, we employ a five-trocar setup using three standard pars plana trocar/cannulas and two limbal anterior trocar/cannulas (Figure 1). The latter two ports are typically necessary in cases of endophthalmitis complicated by significant anterior segment inflammatory reaction and/or media opacity.
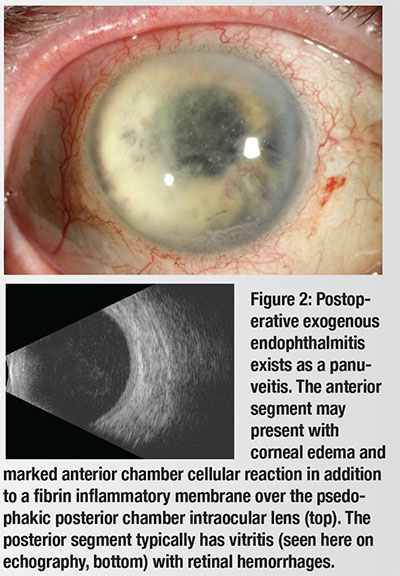 |
| Endophthalmitis: What We Know So Far The most common form of infectious endophthalmitis tends to be exogenous (Figure 2), as opposed to endogenous, mostly following cataract surgery or intravitreal injection. It tends to present acutely within three to 21 days after the procedure. The timing of onset can help in identifying the infectious organism. Coagulase-negative Staphylococcus and Streptococcus, rather than Gram-negative organisms, typically cause acute-onset endophthalmitis within six weeks of an intraocular procedure.7 Chronic or delayed-onset endophthalmitis (beyond six weeks of intraocular surgery) is typically due to Propionibacterium acnes but may also involve Coagulase-negative Staphylococcus or fungi.8 Bleb-associated endophthalmitis can occur months to years after filtering surgery and is most commonly caused by Streptococcus, Haemophilus or Gram-positive organisms. We previously reviewed 10 years of endophthalmitis cases (n = 758) and found Gram-positive organisms to be the causative pathogen in 80 percent of cases.7 Endophthalmitis incidence rates are difficult to determine because studies are usually under-powered, owing to its rare incidence after ocular surgery. The limited number of homogeneous study populations, different surgical techniques and variability in reporting methods make extrapolation of its prevalence difficult. Several reports have documented the evolving incidence of endophthalmitis, with rates varying from 0.03 percent to 0.345 percent.1,3,9-16 Preoperative use of providone iodine anti-sepsis has the strongest evidence as a prophylaxis during intravitreal injection, with a Grade B recommendation.17 In post-cataract surgery endophthalmitis, the value of intraoperative intracameral antibiotics has been vigorously debated, with some authors suggesting they reduce the incidence of this devastating complication.18,19 |
Antibiotic Therapy
Another point of contrast to the EVS concerns systemic antibiotics. Although EVS showed no additional treatment benefit with systemic antibiotics, oral fourth-generation fluoroquinolones like moxifloxacin, which have excellent ocular and vitreous penetration, were not yet available.2-4
In cases of endophthalmitis, a 10- or 14-day course of oral moxifloxacin (400 mg daily) provides additional broad-spectrum coverage with good vitreous penetration. We tend to supplement this in a similar manner with topical fluoroquinolones, steroids and cycloplegia for added anti-microbial, anti-inflammatory and analgesia effects, respectively.
Although bacteria comprise the majority of causative pathogens in postoperative exogenous endophthalmitis, one needs to consider other organisms, such as fungi.4 Additionally, we recently showed Acanthamoeba could cause an atypical postoperative panuveitis in a patient who underwent multiple penetrating keratoplasty surgeries. Our team is one of the first to histologically document Acanthamoeba involvement in all ocular layers with confirmed choroidal involvement.6
This example serves as a reminder for broad consideration when encountering suspected cases of infectious endophthalmitis. Active communication with patients and close follow-up of evolving clinical response are paramount to achieving the best outcomes. RS
Dr. Mandelcorn is an assistant professor of ophthalmology at the University of Toronto.
Dr. Almeida is a Canadian vitreoretinal surgeon with VitreoRetinal Surgery, PA, Minneapolis; Dr. Chin is with Retina Consultants of Southern California in Redlands.
DISCLOSURES: Dr. Almeida disclosed relationships with Allergan, Citrus Therapeutics and Genentech. Dr. Chin disclosed a relationship with Citrus Therapeutics.
REFERENCES
1. Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479-1496.
2. Almeida DR, Miller D, Alfonso EC. Anterior chamber and vitreous concordance in endophthalmitis: implications for prophylaxis. Arch Ophthalmol. 2010;128:1136-1139.
3. Almeida DR, Chin EK, Shah SS, et al. Comparison of microbiology and visual outcomes of patients undergoing small-gauge and 20-gauge vitrectomy for endophthalmitis. Clin Ophthalmol. 2016;10:167-172.
4. Xu K, Almeida DRP, Chin EK, Mahajan VM. Delayed fungal endophthalmitis secondary to Curvularia. AJO Case Reports. 2016;3:1-4.
5. Lemley CA, Han DP. Endophthalmitis: a review of current evaluation and management. Retina 2007;27:662-680.
6. Somani S, Grinbaum A, Slomovi AR. Postoperative endophthalmitis: incidence, predisposing surgery, clinical course and outcome. Can J Ophthalmol. 1997;32(5):303-310.
7. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
8. Moshirfar M, Feiz V, Vitale AT, et al. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series. Ophthalmology. 2007;114:686-691.
9. Miller JJ, Scott IU, Flynn HW Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000-2004): incidence, clinical setting, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139:983-987.
10. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123:613-620.
11. West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the US Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388-1394.
12. Hatch WV, Cernat G, Wong D, Devenyi R, Bell CM. Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology. 2009;116:425-430.
13. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology 2002;109:13-24.
14. Creuzot-Garcher C, Benzenine E, Mariet AS, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: a nationwide study in France from 2005 to 2014. Ophthalmology. 2016;123:1414-1420.
15. Schwartz SG, Grzbowski A, Flynn HW Jr. Antiobiotic prophylaxis: different practice patterns within and outside the United States. Clin Ophthalmol. 2016;10:251-256.
16. Hariprasad SM, Shah GK, Mieler WF, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch Ophthalmol. 2006;124:178-182.
17. Fuller JJ, Lott MN, Henson NM, et al. Vitreal penetration of oral and topical moxifloxacin in humans. Am J Ophthalmol. 2007;143:338-340.
18. Lott MN, Fuller JJ, Hancock HA, et al. Vitreal penetration of oral moxifloxacin in humans. Retina 2008;28:473-476.
19. Mammo Z, Almeida DR, Cunningham MA, Chin EK, Mahajan VB. Acanthamoeba endophthalmitis after recurrent keratitis and nodular scleritis. Retin Cases Brief Rep. 2016 May 5 [Epub ahead of print].




