| Fourth Annual Pipeline Report |
Take-home Points
|
 |
| How the list was compiled
|
The COVID-19 pandemic has slowed clinical trials, but a number of investigative agents in retina continued to move toward commercialization in the past year, setting up 2021 to be a year with many significant readouts.
The past year has seen significant advances in exudative retinal disease treatments using gene therapy and targeting the complement pathway, but no significant approvals.
Two emerging potential blockbusters encountered setbacks in 2020. Brolucizumab (Beovu, Novartis), approved in late 2019, was the subject of an American Society of Retina Specialists’ update alerting members to reports of retinal vasculitis linked to the drug. Abicipar pegol, the designed ankyrin repeat protein (DARPin) therapy that AbbVie inherited with its acquisition of Allergan, failed to gain regulatory approval when the Food and Drug Administration issued a complete response letter that noted the post-administration rate of intraocular inflammation resulted in an unfavorable benefit-risk ratio. Both brolucizumab and abicipar remain in our list, the former because Novartis is seeking an additional indication for diabetic macular edema, the latter because trials are ongoing and AbbVie continues to pursue development.
Three lists this year
Each year the list gets bigger, and this year’s breaks out into three different listings: biologics, steroids and light-activated treatments for exudative disease; gene therapies for exudative disease; and therapies for inherited retinal disease.
This year’s report lists 51 entries, up from 23 last year. Besides the five gene therapy and eight IRD candidates added, 14 entries have been added to the list of biologic and other therapies for exudative disease.
Two candidates have been dropped from the list: AKB-9778 (Aerpio Therapeutics); and DE-122 (Santen/TRACON Pharmaceuticals).
AKB-9778, also known as razuprotafib, is patient self-administered like insulin. The Phase IIb trial in nonproliferative diabetic retinopathy failed to meet its primary endpoint. Sponsor Aerpio Therapeutics is pursuing its development in glaucoma. Santen Pharmaceutical and Tracon Pharmaceuticals last March discontinued development of the carotuximab endoglin antibody DE-122 after discouraging results of a Phase IIa trial in neovascular age-related macular degeneration.
This listing includes only therapies in human trials or soon to be in the clinic.
Abicipar pegol (AbbVie/Molecular Partners)
The SEQUOIA (n=949, Clinicaltrials.gov identifier NCT02462486) and CEDAR (n= 233, NCT02173496) Phase III trials reported rates of intraocular inflammation (IOI) of 15.1 to 15.7 percent in abicipar-treated patients compared with 0 to 0.6 percent in the ranibizumab treatment group.1 Shortly after the FDA CRL, AbbVie and Molecular Partners—they’re collaborating on development—withdrew the European Medicines Agency application for abicipar in nAMD. Trial readouts continued after the CRL, and the collaborating companies say they’re committed to abicipar to fill an unmet need for treatment options in nAMD.
DARPin molecules are derived from naturally occurring binding proteins that consist of repeat sequences with capping structures at each end of the protein. They have three key properties that make them an important investigational class of binding protein for researchers: high binding affinity; low molecular weight; and customizable applications.
The Phase II Maple trial reported an overall IOI rate of 8.9 percent (11 of 123 eyes). A deep analysis of those 11 eyes found that all cases responded to treatment with topical or intraocular steroids and had resolved by the time the study was completed, and that vision improved in most eyes.2 No cases of endophthalmitis or vasculitis were reported. Thomas A. Albini, MD, presenting the data at the Retina Society, said the results showed that a modified manufacturing process for abicipar demonstrated better safety than the formulation used in the Phase III studies.
Aflibercept (Regeneron Pharmaceuticals)
An 8-mg dose of aflibercept is the subject of a number of clinical trials. The Phase II/III PHOTON trial (n=640, NCT04429503) started enrolling patients in June. The Phase III PULSAR trial (n=960, NCT04423718) of patients with age-related vision problems started last summer. A separate Phase II trial (n=100, NCT04126317) in nAMD had started in 2019 and is scheduled for completion later this year.
Meanwhile, the standard 2-mg dose of aflibercept is the subject of the Phase III PANORAMA trial for moderately severe to severe nonproliferative DR without DME (n=402, NCT02718326). The study evaluated two treatment regimens with 2-mg aflibercept: q16 weeks after three monthly doses and one eight-week interval (n=135); and q8 weeks after five monthly doses (n=134). In the q16-week group, 65.2 percent had at least a two-step improvement in Diabetic Retinopathy Severity Score, as did 79 percent in the q8-week group vs. 15 percent in the sham group.3
AKST4290 (Alkahest Inc.)
Formerly known as ALK 4290, AKST4290 is an oral inhibitor of the chemokine C-C motif receptor 3 (CCR3) that blocks the action of eotaxin, an immunomodulatory protein that increases as humans age and contributes to specific age-related diseases. By targeting eotaxin and its downstream effects, AKST4290 may reduce the hallmark inflammation and neovascularization of AMD while also acting more broadly to reduce inflammation associated with many other age-related diseases.
Alkahest initiated a Phase IIb clinical trial, PHTHALO-205 (n=100, NCT04331730), to evaluate efficacy on visual acuity after three loading doses of aflibercept in treatment-naïve nAMD patients. Subjects have been randomized 1:1:1 to receive AKST4290 400 mg b.i.d., AKST4290 800 mg b.i.d. or placebo. Completion is scheduled for April.Biologics, steroids and light-activated treatments for exudative disease in human trials
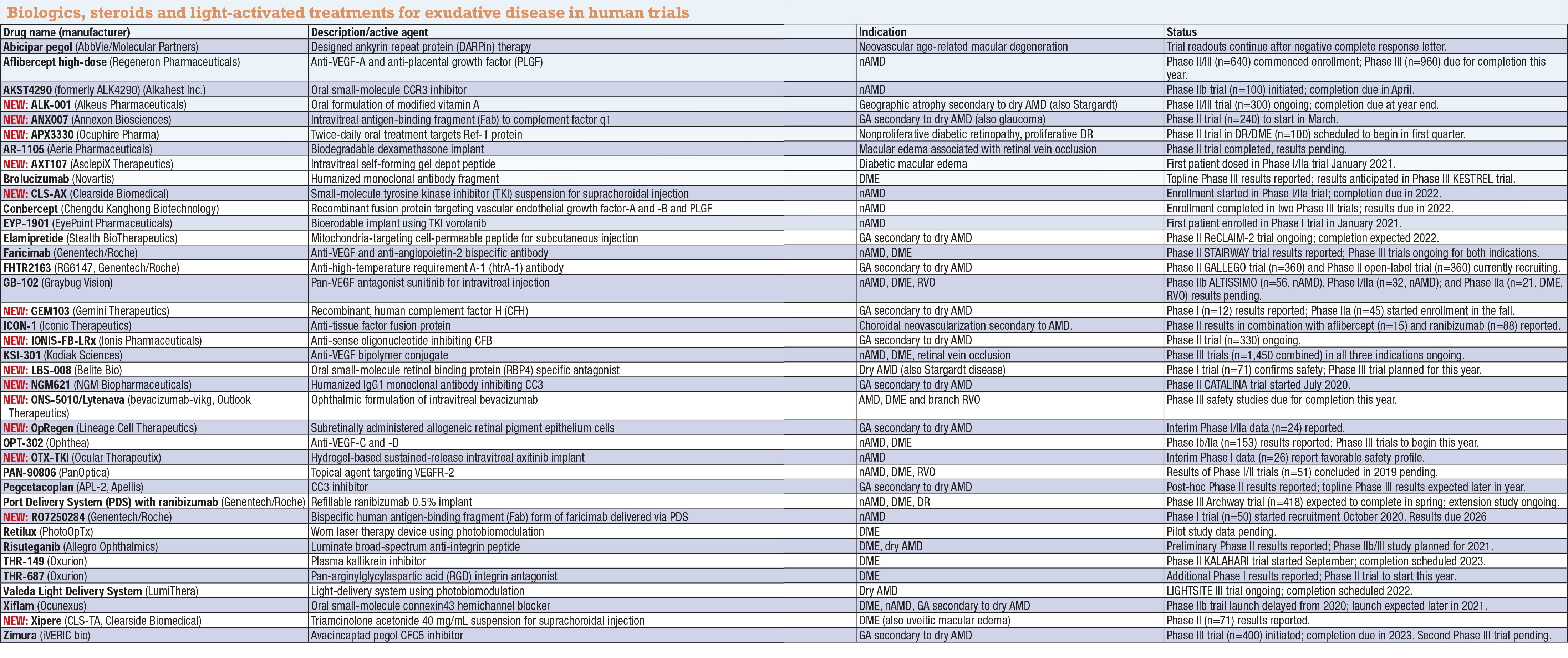 |
NEW: ALK-001 (Alkeus Pharmaceuticals)
A Phase II/III trial (n=300, NCT03845582) of this oral modified form of vitamin A for geography atrophy secondary to dry AMD is recruiting with a completion date set for July. Alkeus is also pursuing concurrent trials in Stargardt disease.
NEW: ANX007 (Annexon Biosciences)
Intravitreal ANX007 is designed to bind to complement factor 1q and inhibit activation of all downstream components of the classical complement cascade, including C3 and C5 without disrupting their normal function in other complement pathways. Based on results of the Phase Ib trial in glaucoma, Annexon has filed to start a Phase II trial in geographic atrophy (n=240, NCT04656561).
APX3330 (Ocuphire)
A Phase II trial started enrollment in January to evaluate the safety of APX3300 in 100 people with moderately severe to severe NPDR and mild PDR (NCT04692688). APX3330 is a twice-daily oral tablet; dosing is five 120-mg tablets daily.
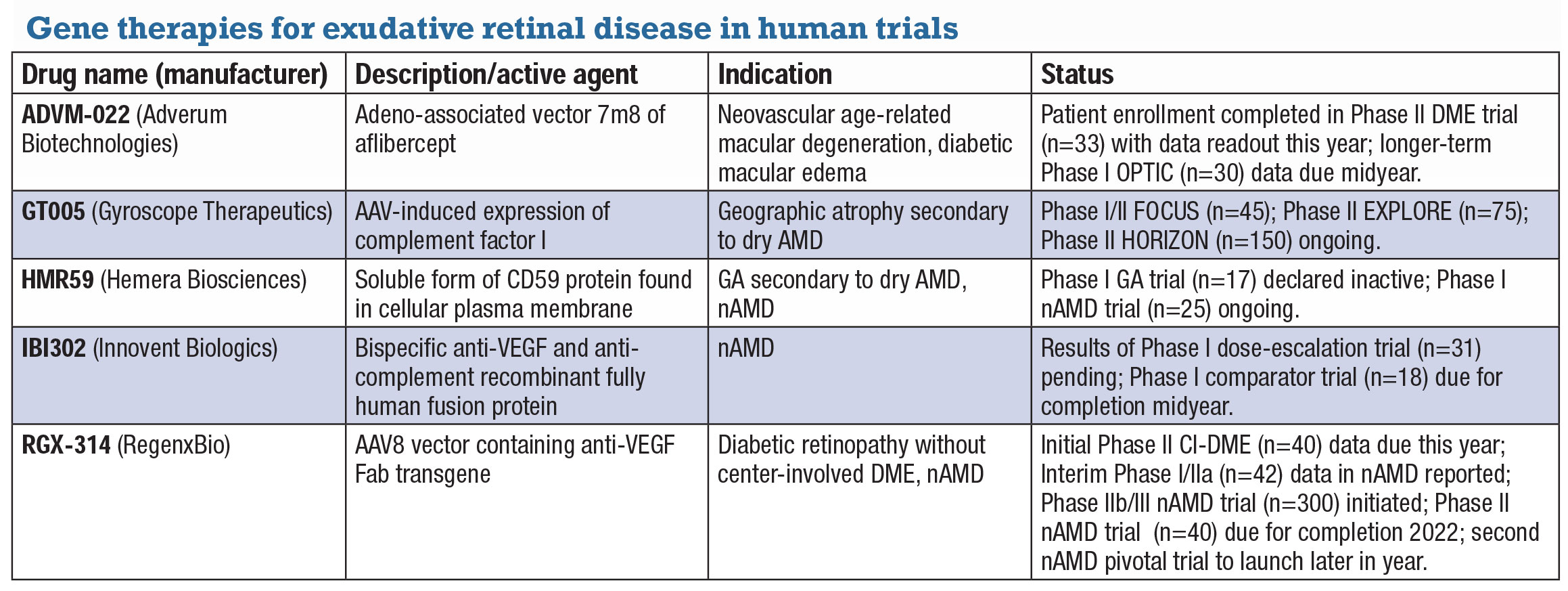
Gene therapies turn to exudative retinal diseasesDevelopers of gene therapy candidates in ocular disease have shifted their focus from inherited retinal diseases, which often have limited treatment populations, to exudative diseases, including geographic atrophy secondary to age-related macular degeneration. They’re also investigating treatments in neovascular AMD and diabetic macular edema to reduce or eliminate the treatment burden of anti-VEGF therapies. The following is a list of investigative gene therapy candidates for exudative retinal disease.
ADVM-022 (Adverum Biotechnologies) ADVM-022 is a single intravitreal injection that uses a propriety adeno-associated vector capsid, AAV.7m8, carrying an aflibercept-coding sequence under the control of a proprietary expression cassette. It’s the subject of two clinical trials in nAMD and one in DME. Partial data from cohorts in the OPTIC Phase I trial (n=30, NCT03748784) demonstrated durability of a single injection out to 92 weeks with no rescue and 99 and 85 percent reductions in annual anti-VEGF injections in high- and low-dose groups, respectively. Adverum says it will present longer-term OPTIC data in the first half of the year and initiate a pivotal trial by midyear. In DME, Adverum says it expects to present data from the INFINITY Phase II (n=33, NCT04418427) trial in the second half of the year, and anticipates launching a pivotal trial in nAMD at midyear.
GT005 (Gyroscope Therapeutics) A one-time therapy delivered subretinally, GT005 is designed to restore balance to an overactive complement system by inducing expression of complement factor I. The Food and Drug Administration last year approved the Orbit subretinal microinjection system and granted fast-track designation for GT005 in GA. GT005 is being evaluated in three trials: Phase I/II FOCUS (n=45, NCT03846193), an open-label dose-escalation safety study of a single injection in GA; and the EXPLORE (n=75, NCT04437368) and HORIZON (n=150, NCT04566445) trials, both Phase II studies evaluating two doses in one injection in GA.
HMR59 is a soluble form of CD59, the protective protein normally found on the cellular plasma membrane. HMR-1001, a Phase I dose-escalation trial (n=17, NCT03144999) in GA, shut down last year. HMR-1002 is a Phase I proof-of-concept study (n=25, NCT03585556) in treatment-naïve patients with new onset nAMD. Patients will be treated with anti-VEGF followed by intravitreal AAVCAGs CD59 a week later. They’ll be followed for a year and receive anti-VEGF monthly as needed. Completion is expected next year.
IBI302 (Innovent Biologics) China-based Innovent describes IBI302 as a bispecific anti-VEGF and anti-complement recombinant fully human fusion protein. The Phase I trial in nAMD (n=31, NCT03814291) of a single intravitreal injection found no serious adverse events or dose-limiting toxicity and demonstrated signs of improved vision and reduction of retinal edema.12 At 28 days, all patients showed improvement in BCVA and central retinal thickness. Treatment duration in some patients lasted up to six weeks. A second Phase I trial (n=18, NCT04370379) is evaluating repeat treatment with high- and low-dose IBI302 with aflibercept as a comparator. Midyear completion is expected.
RGX-314 (RegenxBio) RGX-314 is an AAV8 vector containing a transgene for anti-VEGF Fab that RegenxBio is investigating in diabetic retinopathy and nAMD. ALTITUDE, a Phase II trial (n=40, NCT04567550), is evaluating suprachoroidal delivery of RGX-314 using the SCS Microinjector in DR without center-involved DME. Initial data are due this year. In nAMD, interim Phase I/IIa data (n=42, NCT03066258) demonstrated significant reduction in anti-VEGF injections after RGX-314 subretinal delivery, with 73 percent of patients in one cohort (8/11) remaining anti-VEGF free at a year. 13 Two trials in nAMD are planned. Enrollment started in the Phase IIb/III ATMOSPHERE study (n=300, NCT040704921) comparing RGX-314 and ranibizumab. A smaller Phase II trial, AAVIATE, (n=40, NCT04514653) is evaluating suprachoroidal delivery in nAMD. RegenxBio says it plans to start the second pivotal trial in nAMD in the second half of the year.
|
AR-1105 (Aerie Pharmaceuticals)
This bioerodable intravitreal dexamethasone implant was the subject of positive topline results from a completed Phase II trial in patients with macular edema associated with retinal vein occlusion (n=49, NCT03739593). Aerie reports the results showed positive and sustained treatment effects with two different formulations of AR-1105, with the second formulation demonstrating a duration of up to six months.
In its third-quarter 2020 report, Aerie says it’s evaluating the clinical and regulatory pathway for the agent. Complete results are still pending.
NEW: AXT107 (AsclepiX Therapeutics)
Patient enrollment started in January in the Phase I/IIa CONGO trial to evaluate the safety and bioactivity of AXT107 in patients with DME (n=18, NCT04697758). AXT107 inhibits vascular endothelial growth factor A and VEGF-C, and activates Tie2 as well. The FDA late last year cleared the Investigational New Drug application (IND) for AXT107 for DME, nAMD and macular edema following RVO.
Brolucizumab (Novartis)
Novartis reports topline results of the Phase III KITE study in DME demonstrated non-inferiority vs. aflibercept 2 mg in mean BCVA change after a year (n=361, NCT03481660).
A second study in DME, KESTREL, reported similar outcomes of 6-mg brolucizumab—the dose approved for nAMD—relative to aflibercept (n=571, NCT03481634). More than half the patients in the brolucizumab 6-mg arm were maintained on a three-month dosing interval through year one following the loading phase. KESTREL also evaluated brolucizumab 3 mg. KESSTREL anticipates results in the fall.
NEW: CLS-AX (Clearside Biomedical)
Axitinib is a small-molecule tyrosine kinase inhibitor (TKI) commonly used to treat renal cell carcinoma. CLS-AX is a proprietary suspension of axitinib for suprachoroidal injection. Enrollment started in January of the Phase I/IIa OASIS dose-
escalation trial in nAMD (n=15, NCT04626128). Eligible patients had stable visual acuity following two or more previous anti-VEGF injections. Enrolled patients initially receive aflibercept at the first visit and a single dose of CLS-AX at the second visit one month later. Study completion is expected next year.
Axitinib has intrinsic high potency and pan-VEGF inhibition through receptor blockade.
Conbercept (Chengdu Kanghong Biotechnology)
Conbercept is an anti-VEGF recombinant fusion protein that’s been approved in China since 2013. It targets VEGF-A and -B along with placental growth factor (PLGF). Two Phase III trials, PANDA-1 and PANDA-2, have each enrolled 1,140 patients with nAMD (NCT03577899, NCT03630952). The trials recently completed 36-week primary endpoint visits of enrolled patients and both are scheduled for completion in early 2022. Sponsor Chengdu Kanghong says it expects a global launch in 2023.
EYP-1901 (EyePoint Pharmaceuticals)
EyePoint has dosed the first patient in the Phase I clinical trial of EYP-1901 as a potential twice-yearly, sustained-delivery anti-VEGF treatment in nAMD. EYP-1901 combines the bioerodable Durasert sustained-release insert with vorolanib, a multi-kinase inhibitor that’s shown potential in previous human trials in nAMD as an oral therapy. The trial isn’t listed yet on ClinicalTrials.gov.
Elamipretide (Stealth BioTherapeutics)
Elamipretide is a cell-permeable peptide delivered via a 40-mg subcutaneous injection that targets mitochondrial dysfunction. The Phase II ReCLAIM-2 study in AMD with noncentral GA (n=180; NCT03891875) is ongoing with a completion date set for March 2022.
In the past year, Stealth has pursued nonocular indications for elamipretide, namely cardiomyopathy in Barth syndrome, a genetic disorder characterized by dilated cardiomyopathy, skeletal myopathy, neutropenia and short stature, as well as primary mitochondrial myopathy.
The key endpoint of ReCLAIM-2 is low-luminance BCVA at 48 weeks, with secondary outcomes including change in GA area measured by fundus autofluorescence and/or optical coherence tomography at 48 weeks.
Faricimab (Genentech/Roche)
Faricimab is a bispecific antibody that binds and neutralizes both angiopoietin-2 (Ang-2) and VEGF-A. STAIRWAY (n=76, NCT03038880) was a 52-week Phase II trial of nAMD patients that compared faricimab 6 mg q16 weeks flexible dosing or q12-week fixed dosing, both after four monthly injections, and monthly ranibizumab 0.5 mg. Best-corrected VA improvements in both faricimab groups were comparable with ranibizumab, as was improvement in central subfield thickness.4
Genentech has reported top-line results from two large, global, Phase III trials, TENAYA (n=671, NCT03823287) and LUCERNE (n=658, NCT03923300) comparing faricimab to aflibercept in nAMD. Faricimab injections at intervals of up to q16 weeks achieved comparable vision outcomes to aflibercept, and 45 percent of patients in both studies received faricimab every 16 weeks during the first year. Completion for both is scheduled for late next year.
Parallel Phase III trials, YOSEMITE (n= 940, NCT03622580) and RHINE (n= 951, NCT03622593), are evaluating faricimab for DME. Genentech reported that both studies met their primary endpoints and showed that faricimab at q8 weeks and q16-week flex dosing demonstrated comparable VA gains with aflibercept q8 weeks, and that faricimab was generally well-tolerated with no new safety signals identified. In addition, the Phase III Rhone-X study (n=1,800, NCT04432831) is investigating the long-term effect of faricimab in DME, with completion expected in 2023.
NEW: FHTR2163 (Genentech/Roche)
Also known as RG6147, this antibody, delivered by intravitreal injection, inhibits high-temperature requirement A1 (HtrA1), a serine protease gene associated with GA.5 HtrA1 has also been identified as a major risk factor for wet AMD.6 The Phase II GALLEGO trial (n=360, NCT03972709) of patients with GA is evaluating outcomes over 76 weeks with completion due next year. A separate open-label Phase II trial (n=360, NCT04607148) comparing q4-week and q8-week dosing is due at the end of 2023.
GB-102 (Graybug Vision)
GB-102 is a proprietary microparticle depot formulation of the pan-VEGF inhibitor sunitinib designed to be administered intravitreally twice yearly. Treatment in the Phase IIb ALTISSIMO trial (n=56, NCT03953079) in nAMD concluded in January. Graybug reports that 12-month topline data are expected to be announced in the second quarter and full results later in the year. A second open-label Phase I/IIa trial (n=32, NCT03249740) in nAMD provided evidence of durable biological activity for up to eight months from a single intravitreal injection with minor reports of depot migration.
An ongoing, open-label Phase IIa trial (n=21, NCT04085341) is evaluating GB-102 in DME and macular edema secondary to RVO. A three-month safety analysis reported further evidence demonstrating the safety of the 1-mg dose, with a reduced number of particle migration events compared to the ADAGIO trial, while the rate of drug-related adverse events in the 2-mg arm remained unchanged.
NEW: GEM103 (Gemini Therapeutics)
GEM103 was granted FDA fast-track designation for GA secondary to dry AMD. GEM103 is a recombinant, human complement factor H. After topline Phase I results confirmed the drug’s safety (n=12, NCT04246866), the Phase IIa
ReGAtta study (n=45, NCT04684394) enrolled its first patient last fall. Completion of the trial is scheduled for year end.
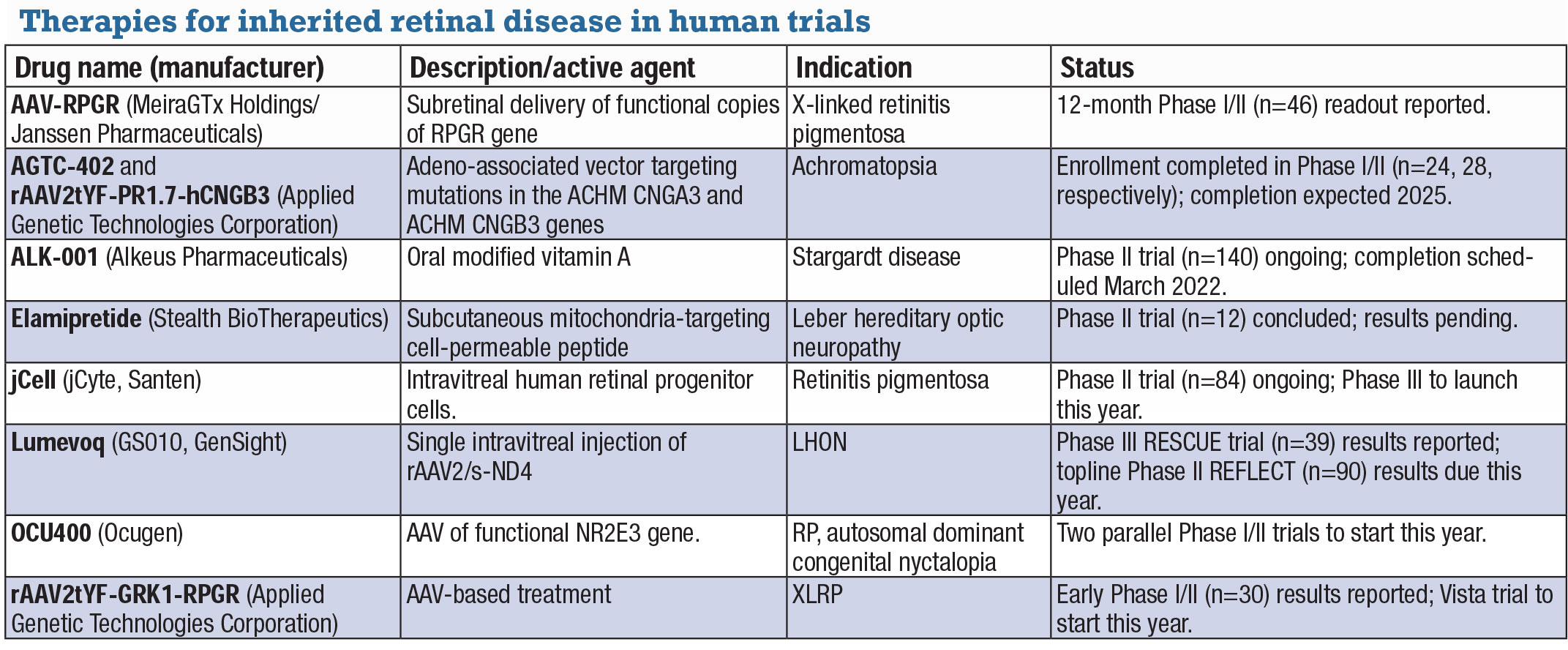
Inherited retinal disease treatments move beyond gene therapiesJust as developers of gene therapy candidates have expanded their research beyond inherited retinal disease and into exudative retinal disease, some non-gene therapy candidates have emerged to treat IRD. The following IRD candidates are in human trials. AAV-RPGR (MeiraGTx Holdings/Janssen Pharmaceuticals) AAV-RPGR is designed to deliver functional copies of the RPGR gene to the subretinal space. Twelve-month data from the ongoing Phase I/II clinical trial (n=46, NCT03252847) in X-linked retinitis pigmentosa (XLRP) reported statistically significant vision improvement in the dose-escalation phase sustained for a year, although the numbers were small: six of seven patients in two dosing cohorts.14 These findings are being evaluated at additional time points.
AGTC-402 and rAAV2tYF-PR1.7-hCNGB3 Enrollment in two Phase I/II trials of gene therapies in achromatopsia have been completed: AGTC-402 for mutations in the ACHM CNGA3 gene (n=24, NCT02935517); and rAAV2tYF-PR1.7-hCNGB3 for mutations in the ACHM CNGB3 gene (n=28, NCT02599922). Early results from dose-escalation cohorts of the trials showed positive signals in improving light discomfort and a favorable safety profile.
ALK-001 (Alkeus Pharmaceuticals) A Phase II trial of this oral-modified form of vitamin A in Stargardt disease (n=140, NCT02402660) is underway. Vitamin A dimers have been linked to vision loss, and ALK-001 is designed to prevent formation of these toxic dimers and replace vitamin A.
Elamipretide (Stealth BioTherapeutics) Elamipretide is a cell-permeable peptide delivered via a 40-mg subcutaneous injection that targets mitochondrial dysfunction. Results are pending of a Phase II study in Leber hereditary optic neuropathy (n=12, NCT02693119).
jCell (jCyte/Santen) jCell is an intravitreal injection of human retinal progenitor cells (hRPC), now in a Phase II trial (n=84, NCT03073733) in RP. The treatment aims to preserve or restore vision independent of the mutated gene causing the disease. In the Phase IIb study, patients received 3 million cells, 6 million cells or a sham treatment in one eye. The high-dose group had a mean improvement in best-corrected visual acuity of 7.43 letters vs. 1.96 and 2.81 letters in the 3-million cell and sham groups. jCyte is planning a Phase III trial this year.
Lumevoq (GS010, GenSight) Lumevoq is a single IVI of rAAV2/2-ND4. The Phase III RESCUE trial (n=39, NCT02652767) in LHON caused by a mutation in the ND4 mitochondrial gene reported 71 percent of patients had >15-letter improvement in at least one eye at 96 weeks.15 GenSight expects approval in Europe later in the year and topline results from the Phase III REFLECT trial (n=90, NCT03293524) in the second quarter.
OCU400 (Ocugen) OCU400 has received orphan drug designation for the treatment of PDE6B gene mutation-associated retinal diseases. OCU400 consists of a functional copy of a nuclear hormone receptor gene, NR2E3, delivered to retina target cells via an adeno-associated viral vector. Expression of NR2E3 within the retina may help reset retinal homeostasis. A potential indication is RP caused by PDE6B mutation autosomal-dominant congenital stationary nyctalopia. Ocugen says it’s planning to initiate two parallel Phase I/II clinical trials this year.
rAAV2tYF-GRK1-RPGR (AGTC) Early results have been reported in the Phase I/II trial of this AAV-based therapy in XLRP (n=30, NCT03316560). A small group in the trial demonstrated improved visual sensitivity and stable or improving vision at 12 months. Last fall AGTC said it expects to initiate enrollment in the Vista trial in the first quarter this year.
|
ICON-1 (Iconic Therapeutics)
Iconic describes ICON-1, also labeled hI-con1 in clinical trials, as a fusion protein that binds to tissue factor overexpressed in the retina and the choroid of patients with AMD.
A Phase II clinical trial for choroidal neovascularization and AMD, called EMERGE (n=88, NCT02358889), found ranibizumab alone to be superior to hI-con1 0.3 mg alone or in combination with ranibizumab for six-month improvement in central subfield thickness, but no significant difference between combination treatment and ranibizumab monotherapy for six-month VA improvement (patients on hI-con1 alone actually lost 2.1 letters at six months).
Results are pending in the second Phase II study (n=15, NCT03452527), called DECO (Dose Exploration and Continuation Option), which evaluated intravitreal ICON-1 0.6 mg in combination with aflibercept 2 mg for CNV in AMD.
NEW: IONIS-FB-LRx (Ionis Pharmaceuticals)
IONIS-FB-LRx is an antisense oligonucleotide (ASO) that inhibits complement factor B gene expression by binding with factor B mRNA. It’s the subject of a Phase II placebo-controlled trial (n=330, NC03815825) in GA that will evaluate change in GA area at week 49. Study completion is expected in late 2022.
KSI-301 (Kodiak Sciences)
KSI-301 is the subject of clinical trials for three indications in exudative disease. In treatment-naïve nAMD, patient recruitment closed last fall in the Phase IIb/III DAZZLE study (n=550, NCT04049266) comparing KSI-301 and aflibercept. Completion is expected in late 2022. The primary endpoint is mean change in BCVA at one year.
In treatment-naïve DME, the Phase III GLEAM (n=450, NCT04611152) and GLIMMER (n=450, NCT04603937) studies are comparing KSI-301 on an individualized dosing regimen of q8 to q24 weeks after three loading doses and aflibercept q8 weeks after five loading doses.
Completion for both is expected toward late 2023. The Phase III BEACON study (n=550, NCT04592419)
is evaluating KSI-301 in patients with treatment-naïve macular edema due to RVO. Completion is expected later next year.
NEW: LBS-008 (Belite Bio)
Belite Bio describes LBS-008 as a first-in-class oral, small-molecule, retinol-binding protein 4 (RBP4) specific antagonist for dry AMD. Results of a Phase I trial (n=71, NCT03734810) confirmed safety and tolerability and that oral administration achieved potentially therapeutic-level target engagement. Belite Bio says it expects to enter a global Phase III trial this year and is seeking an additional indication in Stargardt disease.
NEW: NGM621 (NGM Biopharmaceuticals)
NGM621 is a humanized IgG1 monoclonal antibody engineered to potently inhibit C3. NGM initiated the Phase II CATALINA trial (n=240, NTC04465955) in GA last fall. Completion is expected in 2023.
NEW: ONS-5010/Lytenava (bevacizumab-vikg, Outlook Therapeutics)
ONS-5010/Lytenava (bevacizumab-vikg) is an ophthalmic formulation of bevacizumab. Late last year, Outlook completed enrollment in its open-label Phase III safety study, NORSE THREE (n=195, NCT04516278) in nAMD, DME and branch RVO. Completion is expected this quarter.
A Phase III safety study in nAMD alone (n=227, NCT03834753) is scheduled for completion in the summer. The idea is to have a formulation of bevacizumab ready for injection without the need for repackaging by compounding pharmacies.
NEW: OpRegen (Lineage Cell Therapeutics)
This investigational cell therapy consists of allogeneic retinal pigment epithelium cells administered to the subretinal space for GA resulting from dry AMD. Interim results from a Phase I/IIa trial (n=24, NCT02286089) demonstrated improvement in VA and GA area among some treated patients. The study is scheduled for completion in late 2024.
OPT-302 (Ophthea)
Ophthea says it has “successfully completed” post-Phase II meetings with the FDA on its VEGF-C and -D inhibitor and expects to begin Phase III trials in nAMD early this year.
The ShORe trial would randomize patients to one of three study arms: q4-week treatment with ranibizumab in combination with either OPT-302 2 mg q4 weeks, OPT-302 2 mg on an extended q8-week regimen after three monthly doses, or sham q4 weeks.
The COAST trial would have three treatment arms: aflibercept 2 mg q8 weeks after three monthly loading doses in combination with the same two OPT-302 regimens in ShORe; and sham q4 weeks. Each trial would enroll at least 900 patients with a primary endpoint of mean change in VA after a year.
Results of a separate Phase Ib/IIa trial in DME refractory to anti-VEGF-A therapy were reported over the summer (n=153, NCT03397264). The Phase Ib component (n=9) was a dose-escalation study of OPT-302 in combination with aflibercept.
The Phase IIa trial (n=144) was a dose-expansion study that randomized patients to either OPT-302/aflibercept combination therapy or aflibercept alone. That study reported that 52.8 percent of patients on combination therapy achieved a >5-letter improvement in VA at 12 weeks.
NEW: OTX-TKI (Ocular Therapeutix)
OTX-TKI is a reabsorbable intravitreal implant that delivers the small-molecule TKI axitinib in a sustained-release formulation to the vitreous. Interim Phase I data (n=26, NCT03630315) demonstrated a favorable safety profile and evidence of bioavailability with one patient demonstrating durability up to 11 months without rescue.7
PAN-90806 (PanOptica)
This once-daily topical drop that targets VEGF receptor 2 (VEGFR2) was the subject of Phase I/II trials in nAMD (n=51, NCT03479372), which were reported to have confirmed safety and tolerability. Results haven’t yet been posted.
Pegcetacoplan (APL-2, Apellis)
Eighteen-month data from the Phase Ib APL-2-103 study of pegcetacoplan in patients with advanced GA and low vision found that the growth rate of GA lesions was 52 percent slower in treated vs. untreated eyes.
The Phase I clinical trial (n=12, NCT03777332) assessed the safety of the pegcetacoplan formulation (15 mg/0.1 mL) that’s being used in the Phase III DERBY and OAKS studies (n=600, NCT03525600, NCT03525613), for which topline data are expected later in the year.
The patient population in the Phase Ib clinical trial is similar to those of the DERBY and OAKS studies, but includes patients with more advanced disease, a wider range of baseline lesion size and lower baseline visual acuity.
A post-hoc analysis of the Phase II FILLY trial (n=246, NCT02503332) showed a 39-percent reduction in the rate of progression from nascent GA to GA in patients treated monthly vs. sham.
Port Delivery System (PDS) with ranibizumab (Genentech/Roche)
Results from the Phase III Archway clinical trial (n=418, NCT03677934) reported last summer demonstrated that 98.4 percent of PDS patients went six months without needing additional treatment and achieved vision outcomes equivalent to monthly ranibizumab eye injections.8 A Phase III extension study (n=1,000, NCT03683251) is also evaluating long-term safety and tolerability of PDS over 144 weeks with refill exchanges at q24 or q36 weeks. Completion of Archway is expected this spring. The extension study is scheduled for completion in 2025.
NEW: RO7250284 (Genentech/Roche)
This is a bispecific human antigen-binding fragment (Fab) form of faricimab delivered via PDS that Roche is investigating for nAMD. A Phase I trial (n=50, NCT04567303) started recruiting patients in October 2020. Completion is expected in 2026.
Retilux (PhotoOpTx)
Worn like an eye patch, this device is designed to deliver laser therapy directly to the affected eye.
PhotoOpTx describes photobiomodulation (PBM) as irradiation by light in the 630- to 690-nm range. Data are pending from a pilot study (n=134, NCT03866473) comparing PBM with sham in eyes with center-involved DME and good vision.
Risuteganib (Allegro Ophthalmics)
Results are still pending from a Phase II trial (n=42, NCT03626636) comparing the drug candidate formerly known as luminate with sham for nAMD. Allegro reports that preliminary results showed 48 percent of patients in the risuteganib group vs. 7 percent in the sham group met the primary endpoint of >8 letter gain in BCVA (p= 0.013) and that about 1,200 injections have been given outside the study with an acceptable safety profile.
The company this year is planning a larger Phase IIb/III clinical trial to confirm the Phase II study findings.
THR-149, THR-687 (Oxurion)
THR-149 is a plasma kallikrein inhibitor and THR-687 is a pan-arginylglycylaspartic acid (RGD) integrin antagonist. The indication for both is DME.
THR-149 is the subject of a Phase II trial, KALAHARI (n=122, NCT04527107), which will recruit patients with CI-DME refractory to anti-VEGF. Phase IIa will evaluate the optimal dose level, for which data are expected in midyear. Phase IIb will be a comparison study with aflibercept. Topline results are expected in 2023.
Updated data from the Phase I study of THR-687 (n=12, NCT03666923) confirmed safety and demonstrated early signs of efficacy.9 Following a single injection of the highest dose of THR-687, this activity was maintained at three months with a mean BCVA improvement of 12.5 letters. Oxurion says THR-687 is expected to enter Phase II development this year.
Valeda Light Delivery System (LumiThera)
This device also uses photobiomodulation. The LIGHTSITE III trial (n=96, NCT04065490) in dry AMD is ongoing with completion expected next year. Subjects will receive three PBM treatments a week for three weeks for a total of nine sessions.
LumiThera entered into a collaborative arrangement with Diopsys to conduct a pilot study in dry AMD that uses multi-focal electroretinogram function changes as a primary analysis. The device is approved in Europe.
Xiflam (OcuNexus Therapeutics)
Ocunexus was poised to launch a Phase IIb clinical trial of Xiflam in DME, nAMD and GA last year, but those plans were delayed. Now
Ocunexus says it plans to file an IND application with the FDA in the second quarter and start the Phase II trial in the second half of the year.
Xiflam is an oral small-molecule agent that blocks connexin43 hemichannels that have been shown to be overexpressed in exudative retinal disease.
NEW: Xipere (CLS-TA, Clearside Biomedical)
Xipere, formerly known as CLS-TA, is a proprietary triamcinolone acetonide suspension formulated for Clearside’s suprachoroidal delivery platform. Besides trials in noninfectious uveitis, Xipere has been the subject of the Phase II TYBEE trial in DME (n=71, NCT03126786) comparing Xipere in combination with aflibercept with aflibercept alone.10
The study found that the combination group had statistically significant improvement in CST and needed fewer treatments at 24 weeks, although BCVA changes between groups weren’t statistically significantly different.
Zimura (avacincaptad pegol, IVERIC bio)
Zimura is a C5 inhibitor. Results from the Phase III GATHER1 trial (n=400, NCT04435366) in GA demonstrated that patients in the 2- and 4-mg treatment cohorts had a 27.4- and 27.8-percent reduction in average GA growth over a year, respectively, compared with sham. Iveric bio says a second Phase III trial in GA, known as GATHER2, will evaluate Zimura 2 mg over 24 months. Completion of GATHER1 is expected in 2023. RS
REFERENCES
1. Khurana R. Abcipar for neovascular AMD: Two-year results from CEDAR and SEQUOIA Phase 3 clinical trials. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day 2019; San Francisco, CA; October 11, 2019.
2. Albini T. Abicipar Phase 2 MAPLE trial demonstrates improved safety for patients with nAMD following a modified manufacturing process. Paper Presented at Retina Society 2020 VR Live; September 21, 2020.
3. Boyer E. Intravitreal aflibercept injection for nonproliferative diabetic retinopathy: Results from the PANORAMA study. Paper Presented at Retina Society 2020 VR Live; September 22, 2020.
4. Lad E, Lin H, Silverman D, Basu K, Ruiz CQ, Haskova Z. Effect of dual angiopoietin-2/VEGF-A inhibition with faricimab on macular anatomy in the STAIRWAY Phase 2 study of neovascular age-related macular degeneration (nAMD). Paper Presented at Retina Society 2020 VR Live; September 8, 2020.
5. Ciferri C, Lipari MT, Liang WC, et al. The trimeric serine protease HtrA1 forms a cage-like inhibition complex with an anti-HtrA1 antibody. Biochem J. 2015;472:169-181.
6. DeWan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314;5801:989-992.
7. Boyer D. Safety and biological activity of intravitreal OTX-TKI implant in neovascular age-related macular degeneration (nAMD). Paper presented at American Academy of Ophthalmology Retina Subspecialty Day 2020; November 13, 2020.
8. Campochiaro P. Primary analysis results of the Phase III Archway trial of the Port Delivery System with ranibizumab for patients with neovascular AMD. Paper presented at American Society of Retina Specialists Annual Meeting; July 26, 2020.
9. Khanani A. Results of a Phase I study using the integrin antagonist THR-687 in patients with DME. Paper presented at Angiogenesis, Exudation and Degeneration 2020, Miami, FL; February 8, 2020.
10. Barakat M, Wykoff CC, Gonzalez V, et al. Suprachoroidal CLS-TA plus intravitreal aflibercept for diabetic macular edema: A randomized, double-masked, parallel-design, controlled study. Ophthalmol Retina. 2021;5:60-70.
11. Jafee GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: A randomized pivotal Phase II/III trial. Ophthalmology. Published online August 31, 2020. Available at: https://doi.org/10.1016/j.ophtha.2020.08.027.
12. Wang F, Sun X, Zhang M, Lu S, Qian L. Phase I dose-escalation study of IBI302, a novel, bispecitive Fc-fusion protein targeting VEGF and complement system, in patients with nAMD. Poster presented at American Academy of Ophthalmology 2020; November 13, 2020.
13. Wykoff C. Gene therapy with RGX-314 for neovascular AMD: New results from the ongoing Phase I/IIa study. Paper Presented at Retina Society 2020 VR Live; September 22, 2020.
14. Michaelides M, Besirli C, Khan K, et al. AAV-RPGR gene therapy for RPGR-associated X-linked retinitis pigmentosa: 12-month results from a Phase l/2 clinical trial. Paper presented at American Academy of Ophthalmology virtual meeting; November 13, 2020.
15. Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated with 6 months of disease outset. Ophthalmology. Published online January 12, 2021. https://doi.org/10.1016/j.ophtha.2020.12.012.