Take-home points
|
 |
|
Bios Dr. Wingelaar is a vitreoretinal surgery fellow at The Retina Institute, St. Louis. Dr. Blinder is a partner atThe Retina Institute and professor of clinical ophthalmology and visual sciences at Washington University, St Louis. DISCLOSURES: Dr. Wingelaar has no relevant financial disclosures. Dr. Blinder disclosed relationships with Bausch + Lomb, Regeneron Pharmaceuticals, Genentech, Allergan/AbbVie, Aldeyra Therapeutics, Diopsys, Novo Nordisk and Biogen. |
In treating patients with diabetic macular edema, vascular endothelial growth factor is a popular target and anti-VEGF agents have proven to be very effective at both reducing the degree of DME and improving visual acuity. Corticosteroids also are a sensible agent to use in these patients, given the cascade of proinflammatory cytokines involved with DME. However, the challenge with corticosteroids has been in delivering the medication in the appropriate way to achieve a desirable therapeutic result.
The intravitreal dexamethasone implant (DEX, Ozurdex, Allergan/AbbVie) has overcome many of these challenges, as we learned from the MEAD trial conducted in 2014.1 More recently, our own retrospective analysis of MEAD data demonstrated that the DEX implant has the potential for reducing the severity of diabetic retinopathy and halting or slowing disease progression.2 Here, we report on what our retrospective analysis found and review some important lessons from MEAD about the efficacy of DEX in these patients.
Challenges of corticosteroid therapy
We’ve known that topical corticosteroid agents can be effective, but they don’t always penetrate to the retina to yield an adequate response. Systemic steroids have their own set of risks, the largest being disruption of a patient’s glycemic status to a dangerous level.
A sustained-release intravitreal steroid implant allows for direct delivery to the posterior segment, which can offer a prolonged reduction in retinal inflammatory markers and go a long way toward reigning in uncontrolled DME in the right patient.3,4 The MEAD trial thoroughly investigated using the DEX implant in DME.
MEAD takeaways
MEAD, done in 2014, evaluated the safety and efficacy of the dexamethasone intravitreal implant with dosages of 0.7 mg and 0.35 mg in the treatment of DME.1 The study included patients with a diagnosis of DME for a mean duration of 24.9 months with a baseline visual acuity of 20/50 to 20/200 and a central retinal thickness ≥300 µm, as measured with optical coherence tomography.
Study patients had previously been treated with medical or laser therapy. MEAD was designed as two randomized, multicenter, sham-controlled Phase III trials with identical protocols. With three years of follow-up, the study yielded a wealth of data on the effectiveness of the DEX implant.
Over the study course, both the 0.7- and 0.35-mg DEX implants proved to be superior to sham for achieving the primary study endpoint, defined as improvement in best-corrected visual acuity by ≥15 letters from baseline. Not only was this clinically significant; it was statistically significant.
A larger percentage of patients receiving the 0.7-mg implant reached the primary study endpoint than those receiving the 0.35-mg implant—22.2 vs. 18.4 percent.1 After initial treatment, retreatment in MEAD patients was restricted to no more than every six months. This helped to demonstrate the durability of the implant.
The growing burden of DME DME is one of the most common ailments that we face. It can be burdensome to patients and have a significant impact on their quality of life. As the population continues to age and obesity continues to be a problem, both diabetes and diabetic macular edema will increasingly affect our patients.18 The pathogenesis occurs through alteration of the blood-retinal barrier (BRB), characterized by pericyte loss and endothelial cell-to-cell junction breakdown. With a weakened BRB, other systemic conditions can further the development of DME. Hypertension, heart failure and renal failure are all common comorbidities in patients with diabetes. Both inflammation and oxidative stress also contribute by causing an increase in cytokines and growth factors such as VEGF, angiopoietins, tumor necrosis factor, interleukins and matrix metalloproteinases.19 These factors augment the breakdown of the BRB even further and lead to DME.14,20 The identification of these factors allows for selective therapeutic targets to treat DME. —M.J.W., K.J.B. |
DR regression
Our own retrospective analysis of the MEAD data explored the effects of the DEX implant on Diabetic Retinopathy Severity Scale (DRSS) levels,2 which other trials in DME have used to measure disease progression.
We found the DEX implant has the potential to reduce the DRSS and halt or slow disease progression. We evaluated patients improving one or two steps in DRSS score and converting between nonproliferaitve and proliferative DR. Patients treated with the DEX implant showed a one-step improvement in DRSS scores about two months sooner than sham patients.
Additionally, the DEX implant group achieved a two-step improvement in DRSS score about 1.5 months sooner than the sham group. These findings were statistically significant.
Interestingly enough, when looking at time to conversion from NPDR to PDR, patients who had the DEX implant had a delayed conversion time to PDR by two months compared to sham. The treatment arm also demonstrated a regression from PDR to NPDR 1.5 months faster than those in the sham group.2 These results continue to support the utility and benefit of the DEX implant for patients with DME and severe DR.
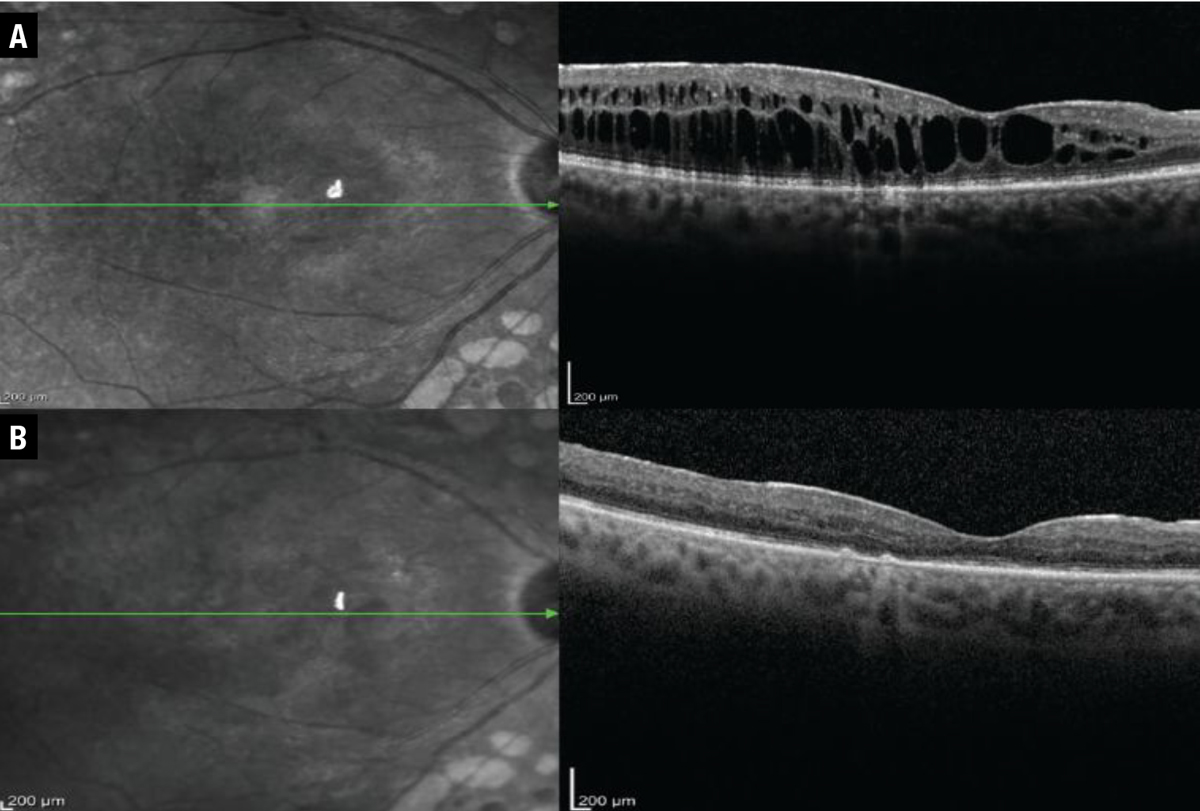 |
| In this patient treated with the dexamethasone intravitreal implant patient, central foveal thickness, as evaluated on optical coherence tomography, improved from (A) 460 µm before treatment with the DEX implant to (B) 210 µm afterward. |
Putting it into practice
Keeping these data in mind, the DEX implant can be a powerful tool when implemented properly. Patients with DME refractory to anti-VEGF therapy or those who aren’t candidates for
anti-VEGF therapy may benefit significantly from intravitreal corticosteroid therapy with the DEX implant, especially pseudophakic patients.5-9
Of course, not all patients will be candidates for the DEX implant, especially if they have a history of glaucoma or have demonstrated an IOP response to prior corticosteroids.
Before inserting the DEX implant, consider first treating the patient with a course of topical steroids to evaluate their risk of a steroid response.
Typically, we treat a patient with a topical corticosteroid for about six weeks before initiating intravitreal corticosteroid therapy. This has the added benefit of possibly demonstrating an improvement in macular edema with topical agents alone, although this response tends to be less than what we would see with the DEX implant.
It’s also critical to discuss with the patient the potential for an IOP increase and cataract development.10,11 In pseudophakic patients, the cataract risk is mitigated, so the DEX implant may be an even more favorable option.12 We’ve seen dramatic improvements in patients’ macular edema when treated with the implant. They also benefit from a reduction in treatment burden due to the implant’s sustained-release mechanism.13,14
We demonstrate one such case (Figure), which shows serial OCTs of a patient before getting the DEX implant and six weeks afterward. This patient’s central foveal thickness improved from 460 to 210 µm and visual acuity improved from 20/50 to 20/30 Snellen equivalent. This patient was minimally responsive to previous anti-VEGF therapy, but tolerated the DEX implant well and didn’t go on to develop an IOP response.
Our DEX protocol
In our practice the DEX implant is a powerful tool when treating DME. When a new patient presents with visually significant macular edema, we start with anti-VEGF therapy or topical corticosteroid drops. In milder cases, we lean toward topical corticosteroid therapy. We monitor the patient closely and bring them in at approximately six weeks to assess their treatment response.
With anti-VEGF therapy, we give patients one or two more injections, depending on their response. If the macular edema exhibits little to no response, we either give another anti-VEGF injection or start the patient on topical drops along with an additional anti-VEGF injection. At this visit, we also discuss the DEX implant with the patient as a treatment option.
The patient comes back in six to eight weeks and if the treatment response remains suboptimal and their IOP hasn’t shown a response to the topical corticosteroids, we start treatment with the DEX implant. The patient returns again in six to eight weeks, when we evaluate the treatment response, paying careful attention to the patient’s IOP to ensure a significant elevation hasn’t occurred.
Patients who have been on long-term anti-VEGF therapy may develop a refractory response to continued treatment. In these instances, the DEX implant also proves to be a valuable treatment option. We manage these patients in a similar fashion: with a trial of topical corticosteroids to assess IOP response and then the DEX implant on follow-up. We can give them supplemental anti-VEGF therapy concurrently to optimize their treatment response and reduce DME. The sustained-release therapy that the DEX implant provides is a significant advantage, as one injection leads to continued control with supplemental therapy available for breakthrough edema.
 |
Managing cataract, IOP outcomes
Cataract development and IOP increase were the primary adverse events in MEAD patients treated with the DEX implant. Some patients ended up losing vision after one year due to the development of cataract, but then had their vision restored following cataract extraction.
Despite cataract formation, at year three study patients receiving the DEX implant had significant improvement in BCVA over the sham group, which indicated that even with a cataract these patients still do well.
In fact, having the DEX implant on board seemed to yield a protective effect against an increase in DME following cataract surgery.15 Upon subgroup analysis, the 0.7-mg implant, currently the marketed dose, proved to be more effective in treating DME, with a safety profile similar to the 0.35-mg device.16
Patients receiving the DEX implant were more likely to have an IOP increase ≥10 mmHg than the sham group—27.7 percent in the 0.7-mg group vs. 24.9 percent in the 0.35-mg group. Fortunately, topical medications controlled IOP in most of these patients. Only three patients—two in the 0.7-mg group and one in the 0.35-mg group—required incisional surgery to correct their IOP rise.
Thus, MEAD provided supportive evidence for the use of the DEX implant in the management of DME. Up to one-third of trial patients achieved vision of 20/40 or better after their first treatment, which appeared to be sustained after correcting for the confounding effect of cataract development.1,17
Bottom line
DME poses a significant burden to a large number of patients. Early intervention with the DEX implant can significantly reduce the degree of macular edema, improve visual acuity and potentially scale back the degree of DR, while at the same time reducing the treatment burden of frequent intravitreal injections. RS
References
1. Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914.
2. Blinder K, Wykoff C, Ferencz J, et al. Dexamethasone implant appears to slow diabetic retinopathy progression: A retrospective analysis of the MEAD study. Paper presented at The Retina Society 54th annual scientific meeting; Chicago, IL; September 29, 2021.
3. Browning D, Stewart M, Lee C. Diabetic macular edema: Evidence-based management. Indian J Ophthalmol. 2018;66:1736-1750.
4. Figueira J, Henriques J, Carneiro Â, et al. Guidelines for the management of center-involving diabetic macular edema: Treatment options and patient monitorization. Clin Ophthalmol. 2021;15:3221-3230.
5. Ehlers JP, Yeh S, Maguire MG, et al. Intravitreal pharmacotherapies for diabetic macular edema. Ophthalmology. 2022;129:88-99.
6. Teja S, Sawatzky L, Wiens T, Maberley D, Ma P. Ozurdex for refractory macular edema secondary to diabetes, vein occlusion, uveitis and pseudophakia. Can J Ophthalmol. 2019;54:540-547.
7. Wang JK, Huang TL, Hsu YR, Chang PY. Effect of dexamethasone intravitreal implant for refractory and treatment-naive diabetic macular edema in Taiwanese patients. J Chin Med Assoc. 2021;84:326-330.
8. Al-Latayfeh M, Abdel Rahman M, Shatnawi R. Outcome of single dexamethasone implant injection in the treatment of persistent diabetic macular edema after anti-VEGF treatment: Real-life data from a tertiary hospital in Jordan. Clin Ophthalmol. 2021;15:1285-1291.
9. Neves P, Ornelas M, Matias I, et al. Dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema: Real-world data versus clinical trials outcomes. Int J Ophthalmol. 2021;14:1571-1580.
10. Schwartz S, Scott I, Stewart M, Flynn Jr. H. Update on corticosteroids for diabetic macular edema. Clin Ophthalmol. 2016;10:1723-1730.
11. Urbani M, Topcic IG, Matovic K. Visual outcomes in patients with diabetic macular edema treated with dexamethasone implant in routine clinical practice. Acta Clin Croat. 2021;60:602-608.
12. Karti O, Saatci AO. Place of intravitreal dexamethasone implant in the treatment armamentarium of diabetic macular edema. World J Diabetes. 2021;12:1220-1232.
13. Nicolò M, Musetti D, Marenco M, et al. Real-life management of diabetic macular edema with dexamethasone intravitreal implant: A retrospective analysis of long-term clinical outcomes. J Ophthalmol. 2020;4860743.
14.Chawan-Saad J, Wu M, Wu A, Wu L. Corticosteroids for diabetic macular edema. Taiwan J Ophthalmol. 2019;9:233-242.
15. He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018;18:121.
16. Augustin AJ, Kuppermann B, Lanzetta P, et al; for the Ozurdex MEAD Study Group. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: Subgroup analysis of the MEAD study. BMC Ophthalmol. 2015;15:150.
17. Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year Phase III trials. Br J Ophthalmol. 2016;100:796-801.
18. Tan GS, Cheung N, Simó R, Cheung GCM, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5:143-155.
19. Noma H, Yasuda K, Shimura M. Involvement of cytokines in the pathogenesis of diabetic macular edema. Int J Mol Sci. 2021;22:3427.
20. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375-1394.



