ABOUT THE AUTHORS | |
 | Drs. Steinle (top) and Pieramici are with California Retina Consultants and Research Foundation, Santa Barbara. Dr. Pieramici is a member of the DRCR.net Protocol T writing committee. |
 | |
Protocol T is the first trial to compare the efficacy and safety of the commercially available anti-VEGF drugs used in the treatment of DME: ranibizumab (Lucentis, Genentech), bevacizumab (Avastin, Genentech) and aflibercept (Eylea, Regeneron). Ranibizumab and aflibercept have been approved for DME by the Food and Drug Administration (FDA), whereas bevacizumab is widely used off-label for DME. (In fact, bevacizumab is not FDA-approved for any ocular condition.)
DRCR.net Protocol 1 confirmed the significant cost differences between these agents—Medicare allowable charges range from $1,961 for 2 mg aflibercept to $1,189 for 0.3 mg ranibizumab to $67 for 1.25 mg bevacizumab—but did not answer to any differences in efficacy or safety.
Impetus for Protocol T
Determining a difference between the agents was the impetus for the DRCR.net Protocol T comparative trial. In the retina community, Protocol T has been dubbed the “CATT study for DME.” However, Protocol T compared three drugs for DME whereas CATT—the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT)—focused on two drugs and dosing strategies (modified PRN versus monthly therapy).2 Here, we review the findings of DRCR.net Protocol T and explore how they can be applied in the clinic.Eighty-nine clinical sites in the United States participated in Protocol T, enrolling 660 adults with either type 1 or type 2 diabetes and DME involving the central macula on optical coherence tomography (OCT). Participants were 18 years or older, with vision of 20/32 to 20/320.
Patients did not have to be naive to treatment, but they could not have received anti-VEGF therapy within the last year. Masked participants were treated with 0.05 mL of either 2 mg aflibercept, 1.25 mg bevacizumab or 0.3 mg ranibizumab, with a primary endpoint of change in visual acuity at one year, with follow-up planned through two years. One eye of each subject was assigned randomly with equal probability to one of the treatment groups.3
The Population Burden of Diabetes and Genesis of anti-VEGF Therapy |
| Diabetes continues to be a growing concern for the American health-care system as its prevalence continues to rise. From 1980 through 2011, the crude prevalence of diagnosed diabetes increased 176 percent in the United States (from 2.5 to 6.9 percent of the population).11 Some statistical models predict that one-third of Americans will have diabetes by 2050.12 Because diabetes is often a disease of working-age adults, it is a tremendous burden on not only the health-care system but also the labor force. In 2011, 63 percent of the adult cases diagnosed within the past year were in individuals ages 40–64 years; and 16% were diagnosed in those ages 18–39 years.13 Approximately half of these diabetic patients will develop retinopathy, with diabetic macular edema being the number one reason for vision loss.14 Thirty years have passed since the landmark Early Treatment Diabetic Retinopathy Study (ETDRS) Report Number 1 was published in 1985, which provided longstanding guidelines for laser photocoagulation use in patients with clinically significant DME.10 Corticosteroids have also been investigated in the treatment of DME, as triamcinolone,15 fluocinolone16 and dexamethasone17 have all been used as intravitreal therapies for DME. Recent years have ushered in the era of anti-VEGF drugs for treatment of DME. Well-designed, multicenter clinical trials have shown the benefits of these drugs in the treatment of DME: the RISE/RIDE studies6 of ranibizumab; the BOLT study18 of bevacizumab; and the VISTA/VIVID Studies19 of aflibercept. The DRCR.net Protocol I study demonstrated the efficacy and superiority of ranibizumab with immediate or deferred laser versus laser alone in the treatment of center-involved DME. Protocol I also revealed that similar visual and anatomic outcomes could be achieved with a PRN (as needed) protocol that reduced the burden of treatment compared to monthly therapy.20,21 It became very apparent to investigators and clinicians that anti-VEGF therapy was superior to the then “standard of care” for DME—laser photocoagulation. |
Vision Outcomes
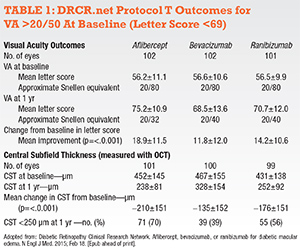
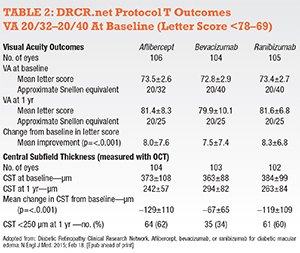
The topline DRCR.net Protocol T results revealed improvement in vision from baseline to one year with all three drugs (Tables 1 and 2, pages 20 and 21). Improvement was greatest with aflibercept (+13 letters) than ranibizumab (+11 letters) or bevacizumab (+10 letters), a statistically significant mean difference of 2-3 letters at one year.This difference appeared to be driven by baseline vision. Half of the study participants had VA of approximately 20/40 or 20/32. In these patients, the mean letter score improvement was +8.3 with ranibizumab, +8.0 with aflibercept and +7.5 with bevacizumab (each pairwise comparison p>0.5).
However, when initial visual acuity was 20/50 or worse, the mean letter improvement was +18.9 with aflibercept, +14.2 with ranibizumab and +11.8 with bevacizumab (p values: aflibercept-bevacizumab <0.001, aflibercept-ranibizumab = 0.003, ranibizumab-bevacizumab = 0.21). OCT central thickness and vision outcomes showed a similar effect favoring aflibercept when baseline retinal thickness was >400 µm.
More injections or other treatments did not drive this relative benefit of aflibercept. In fact, the aflibercept group had one fewer median number of injections9 than the ranibizumab or bevacizumab groups (p=0.045).10 Laser was also performed less often with aflibercept (37 percent) than ranibizumab (46 percent) or bevacizumab (56 percent) (p<0.001).
Anatomic Outcomes
The anatomic results paralleled the vision results, with the greatest benefit on reduction in retinal edema seen in the patients treated with aflibercept. At one year, central OCT thickness decreased on average by 169 µm (aflibercept), 147 µm (ranibizumab) and 101 µm (bevacizumab).Overall, aflibercept reduced retinal edema better than the other two agents (p<0.001) and ranibizumab outperformed bevacizumab (p<0.001). This held true regardless of the presenting vision.
A few key points are worth emphasizing based on the trial results:
• First, all three agents, on average, improved vision and reduced retinal thickness in patients with center-involved DME. The benefits of treatment could be seen as early as four weeks in some patients, but not all patients derived a benefit.
• Second, when patients presented with relatively good vision (20/40 or better), all three drugs resulted in similar vision outcomes at one year, although the anatomic results were better with aflibercept and ranibizumab. Studies have reported that 75 percent of patients with DME present with visual acuity of 20/40 or better.4,5 Since VAs reported in the study were standardized vision measurements, it is not exactly clear how this translates into the vision measured in our clinics. Standardized refracted visions can be several lines of vision better than typical Snellen visions.
 |
 |
Viewed in another way, the chance of three lines of vision improvement in patients with VA of 20/50 or less at baseline was 63 percent greater with aflibercept compared to bevacizumab, and 34 percent greater with aflibercept compared to ranibizumab. Finally, it is important to realize that these represent one-year results to date (and DME is a chronic condition). A planned secondary outcome measure in Protocol T will include change in visual acuity at two years.3 The second-year data will provide insight into the efficacy and safety of extended therapy and perhaps a better understanding of the relative long-term treatment burden of these agents.
Questions About Dosing
Some may wonder whether the 0.5 mg dose of ranibizumab might have performed better than the 0.3 mg dose that was used in Protocol T. We will never know for sure, but data from the RISE and RIDE studies6 found no significant difference between the 0.3 and 0.5 mg doses in terms of efficacy. On the other hand, some smaller studies have demonstrated additional benefit on vision and anatomy in some DME patients when switched to higher dosages of ranibizumab.7A second dosing issue concerns the preparation of bevacizumab. The bevacizumab used in Protocol T was repackaged at a central pharmacy into single-use vials. These vials underwent independent testing for sterility, purity and potency prior to use.1 Although somewhat unlikely to fully account for differences seen with bevacizumab in this study, significant variability of bevacizumab obtained from compounding pharmacies has been reported and needs to be considered in our “real world patients”.8
The Safety Issue
With regards to safety, no differences in intraocular inflammation were noted among the three groups, and endophthalmitis was rare (occurring in 0.02 percent of injections). Rates of serious adverse events, deaths, hospitalizations and major cardiovascular events were similar in the three treatment groups. However, a post hoc analysis did find a small but statistically significant increase in reported cardiac and/or vascular adverse events in the ranibizumab group, although it is possible that this finding was a chance event.The relatively small number of patients in each treatment group (aflibercept, 224; bevacizumab, 218; and ranibizumab, 218) is unlikely to manifest differences in rare serious adverse events. These three agents have known differences in vivo in regards to their systemic pharmacokinetic profiles,9 so the continued monitoring of patients for systemic adverse events is prudent, especially in more at-risk DME patients, many of whom, such as those on dialysis, were ineligible for this and other Phase III clinical trials for DME.
The results of clinical trials should serve as guidelines for clinical care. The study results reflect population average outcomes; much individual variability underlies these findings. When considering the individual patient, these data can be instructive, but many additional issues, including cost, factor in the decision of which agent or method of treatment to utilize initially. In addition, this study did not address the benefits of a treatment strategy that begins with one anti-VEGF agent and then switches to another if the result is less than desirable.
The DRCR.net should be commended for the tedious work involved in seeing Protocol T through to completion. It will remain an important study for many years. As retina specialists, it is an exciting time for our ever-growing diabetic patient population. As opposed to the
ETDRS era of reducing the rate of vision loss in DME,10 it is thrilling to offer our patients three agents that offer the chance for significant vision gains. The debate as to how to best use these three agents in DME from the standpoints of treatment burden, cost, efficacy and safety will certainly provide hours of discussion at the upcoming meetings this year. RS
Disclosures: Dr. Pieramici disclosed he has been a consultant and adviser to Genentech and has received research funding from Genentech and Regeneron. Dr. Steinle is a speaker for Regeneron and ThromboGenics and receives research funding from Genentech.
References
1. The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; Feb 18. [Epub ahead of print].2. Martin DF, Maguire MG, Ying GS, et al, CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897-1908.
3. ClinicalTrials.gov. Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumab, and Ranibizumab for DME (Protocol T). Available at: https://clinicaltrials.gov/ct2/show/study/NCT01627249. Accessed February 10, 2015.
4. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113:1144-1155.
5. Martin DR, Maguire MG. Treatment choice for diabetic macular edema. N Engl J Med. 2015. Feb 18. [Epub ahead of print]
6. Brown DM, Nguyen QD, Marcus DM. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022.
7. Dhoot DS, Pieramici DJ, Nasir M, et al. Residual edema evaluation with ranibizumab 0.5 mg and 2.0 mg formulations for diabetic macular edema (REEF study). Eye (Lond). 2015; Jan. 30 [Epub ahead of print]
8. Yannuzzi NA, Klufas MA, Quach L, et al. Evaluation of compounded bevacizumab prepared for intravitreal injection. JAMA Ophthalmol. 2015; 133:32-39.
9. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014; 98:1636-1641.
10. Early Treatment Diabetic Retinopathy Study Research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796-1806.
11. Centers for Disease Control and Prevention. Crude and Age-Adjusted Rate per 100 of Civilian, Noninstitutionalized Population with Diagnosed Diabetes, United States, 1980–2011. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/figage.htm. Accessed February 26, 2015.
12. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8: 29.
13. Centers for Disease Control and Prevention. Distribution of Age at Diagnosis of Diabetes Among Adult Incident Cases Aged 18–79 Years, United States, 2011. Available at: http://www.cdc.gov/diabetes/statistics/age/fig1.htm. Accessed February 15, 2015.
14. Varma R Bressler N, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132: 1334-1340.
15. Beck RW, Ewards AR, Aiello LP, et al. Diabetic Retinopathy Clinical Research Network (DRCR.net) Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251.
16. Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010; 117: 1393-1399 e3.
17. Boyer DS, Yoon YH Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914.
18. Rajendram R, Fraser-Bell S, Kaines A. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012; 130:972-979.
19 Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254.
20. Elman, MJ, Ayala A, Bressler NM, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35.
21. Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375-381.



