 |
 |
Acute intraocular pressure increases are a well-known common complication of intravitreal anti-VEGF injections. A study in Italy found that 88.9 percent of eyes had an IOP of greater than 30 mmHg directly following injection of ranibizumab (Lucentis, Genentech/Roche).1 A study conducted by Judy Kim, MD, and colleagues at the Medical College of Wisconsin found that IOP rises to a mean of 44 mmHg immediately after intravitreal injection, then quickly declines to below 30 mmHg within 15 minutes.2 In both of these studies, IOP approached normal levels 30 to 60 minutes post-injection for a majority of patients.
Chronic IOP change
A recent analysis using the IRIS registry has found a small but statistically significant decrease in IOP with use of
anti-VEGF injections. Conducted by Elizabeth Atchison, MD, and colleagues, the study analyzed 23,776 unique patients from the IRIS registry who had been diagnosed with neovascular age-related macular degeneration, diabetic macular edema, branch retinal vein occlusion or central retinal vein occlusion.3 Patients received injections of bevacizumab (Avastin, Genentech/Roche, 56 percent), ranibizumab (25 percent) or aflibercept (Eylea, Regeneron Pharmaceuticals, 19 percent) in the right eye but no treatment in the left. Only patients with at least 12 injections of the specified anti-VEGF were examined.
All subgroups of patients showed a small mean decrease in IOP from the baseline to the last injection. On average, the bevacizumab patients had a 1.2- mmHg decrease in IOP in the treated eye compared to aflibercept with a 0.5-mmHg decrease. The change for patients on ranibizumab was lower—a 0.2-mmHg decrease. Average fellow-eye change in IOP for all groups was a 0.2-mmHg decrease. While these changes are all statistically significant, the small mean decrease may not be of significant clinical importance.
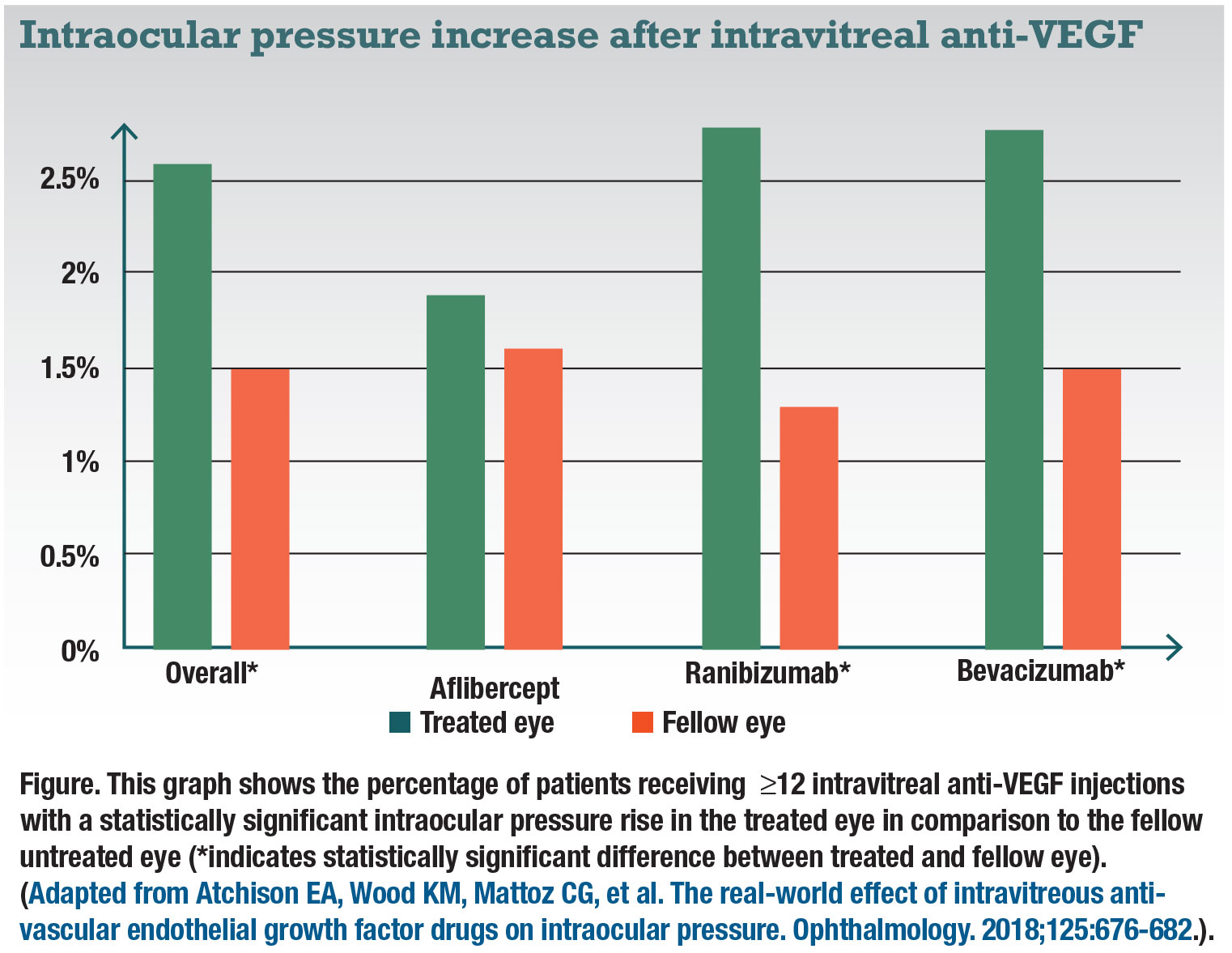
On the other hand, incidents of clinically significant IOP rises, defined by the researchers as an increase of ≥6 mmHg from baseline resulting in an IOP ≥21 mmHg for were 2.6 percent overall vs. 1.5 percent in the fellow eye (Figure). The difference in IOP between treated and untreated fellow eyes was statistically significant for bevacizumab and ranibizumab, but not aflibercept. However, it’s important to note these numbers don’t account for the use of IOP-lowering medications, such as glaucoma drops.
Concern about increased IOP
While this study lacks the structure of prospective clinical trials, its use of
real-world data reflects current clinical practice. The finding of decreased IOP from anti-VEGF injections, while proven statistically significant, is small and likely not of clinical importance. However, the small percentage of patients that experience an increase in IOP could be of clinical significance.
Currently, the exact mechanism that causes this adverse reaction is still under investigation. Additionally, the finding that aflibercept didn’t create any significant increase in IOP in a subgroup of patients compared to bevacizumab and ranibizumab requires further study. For example, could the authors now choose the left eye as the primary eye and run the same statistics to see if the same outcome could be achieved?
 |
 |
How anti-VEGF may influence IOP
Important differences between bevacizumab, ranibizumab and aflibercept lie in their pharmacodynamics, mechanisms of action and targets. All three drugs are antagonists that bind to the active site of human vascular endothelial growth factor A inhibiting the ligands (VEGFR-1 and VEGFR-2) from binding to their endothelial receptors. Aflibercept has the highest binding affinity of the currently used
anti-VEGF drugs.
While ranibizumab and bevacizumab only target VEGF-A, aflibercept targets placenta growth factor and VEGF-B as well.4-6 The half-life of aflibercept is estimated to be six days vs. nine days for ranibizumab and bevacizumab.7 All of these chemical differences could be contributing factors as to why aflibercept does not exhibit the same rate of chronic IOP rise.
Nitric oxide pathway
Aaron Ricca, MD, and colleagues at the University of Arkansas, suggested a physiologic vascular hypothesis for IOP increase arises from alteration of the nitric oxide pathway,8 which is known to relax smooth muscle and endothelial cells. Components of the nitric-oxide pathway have been identified in the anterior chamber. Nitric oxide has been shown to increase anterior chamber aqueous outflow by decreasing trabeculocyte size and vasodilating Schlemm’s canal.
Through its oncologic applications,
anti-VEGF is known to disrupt the normal nitric oxide signaling pathway. When used systemically, anti-VEGF therapy is known to increase arterial hypertension through this mechanism. Taken together, this suggests that increases in IOP may be related to anti-VEGF interaction with nitric oxide physiology.
 |
Genetics and IOP
A potential genetic basis for increased IOP after anti-VEGF therapy has been identified. Researchers in Australia and Europe examined 134 patients that received ranibizumab and identified three polymorphisms of the CD36 gene that were associated with significant IOP increase after therapeutic injection.9 One of the polymorphisms, rs1049673, was associated with pronounced IOP rise (IOP > 25 mmHg).
More connections between genetic and physiological factors are emerging. A National Institutes of Health study has demonstrated thrombospondin-1 inhibition of nitric oxide signaling via CD36.10 Richard Morshedi, MD, and colleagues proposed a clearer mechanism: Anti-VEGF upregulates nitric oxide synthase, decreasing nitric oxide in the anterior chamber and creating decreased trabecular meshwork outflow, thereby increasing IOP.¹¹
Bottom line
Further clinical studies need to be carried out to better understand the complete mechanism responsible for chronic IOP increases as a complication of anti-VEGF injections. RS
REFERENCES
1. Gismondi M, Salati C, Salvetat ML, Zeppieri M, Brusini P. Short-term effect of intravitreal injection of ranibizumab (Lucentis) on intraocular pressure. J Glaucoma. 2009;18:658-661.
2. Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146:930-934e.
3. Atchison EA, Wood KM, Mattoz CG, Barry CN, Lum F, MacCumber MW. The real-world effect of intravitreous anti-vascular endothelial growth factor drugs on intraocular pressure. Ophthalmology. 2018;125:676-682
4. Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–1158.
5. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870.
6. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400.
7. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–1858.
8. Ricca AM, Morshedi RG, Wirostko BM. High intraocular pressure following anti-vascular endothelial growth factor therapy: proposed pathophysiology due to altered nitric oxide metabolism. J Ocul Pharmacol Ther. 2015;31:2-10.
9. Matuskova V, Balcar V, Khan N, et al. CD36 gene is associated with intraocular pressure elevation after intravitreal application of anti-VEGF agents in patients with age-related macular degeneration: Implication for the safety of the therapy. Ophthalmic Genet. 2018;39:4-10.
10. Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415
11. Morshedi RG, Ricca AM, Wirostko BM. Ocular hypertension following intravitreal anti-vascular endothelial growth factor therapy: review of the literature and possible role of nitric oxide. J Glaucoma. 2016;25:291–300.



