
 |
This article describes the role integrins have in angiogenesis, inflammation and neovascularization, and discusses the clinical trials of the three investigational drugs targeting the integrin pathway.
Integrins’ structure and function
Integrins are transmembrane receptors that are heterodimers, which means they consist of both α and β subunits. They mediate cell-to-cell and cell-to-extracellular interactions, the latter in the retinal matrix.1,2 Integrins modulate cell signaling and are involved in various biological pathways. They’ve been implicated in various eye diseases, including cornea neovascularization; glaucoma and dry eye, along with both dry and wet AMD and diabetic retinopathy.3,4
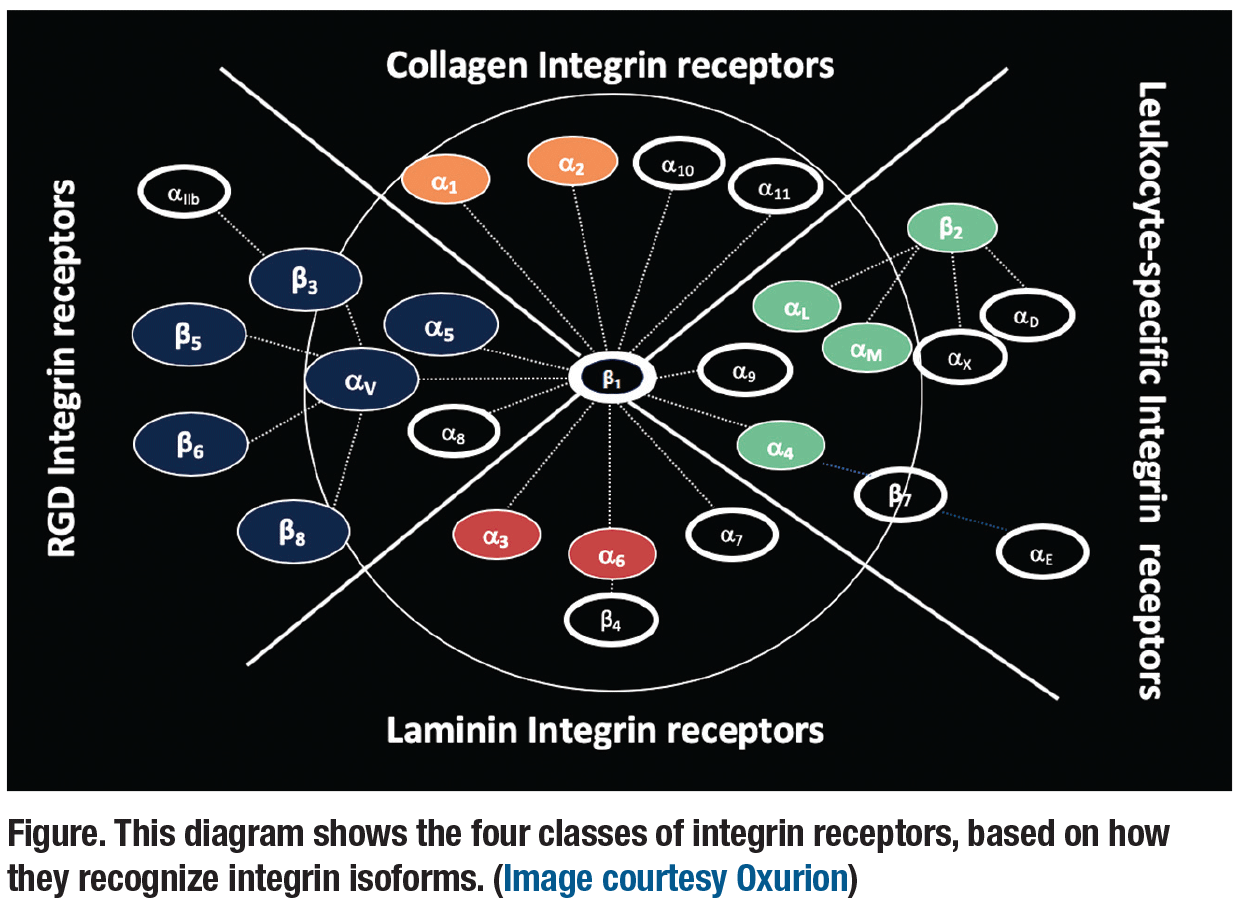
There are four classes of integrin receptors, classified based on how they recognize integrin isoforms (Figure). They are:
- functional arginine-glycine-aspartic acid (RGD) integrin receptors;
- collagen integrin receptors;
- leukocyte-specific integrin receptors; and
- laminin integrin receptors.
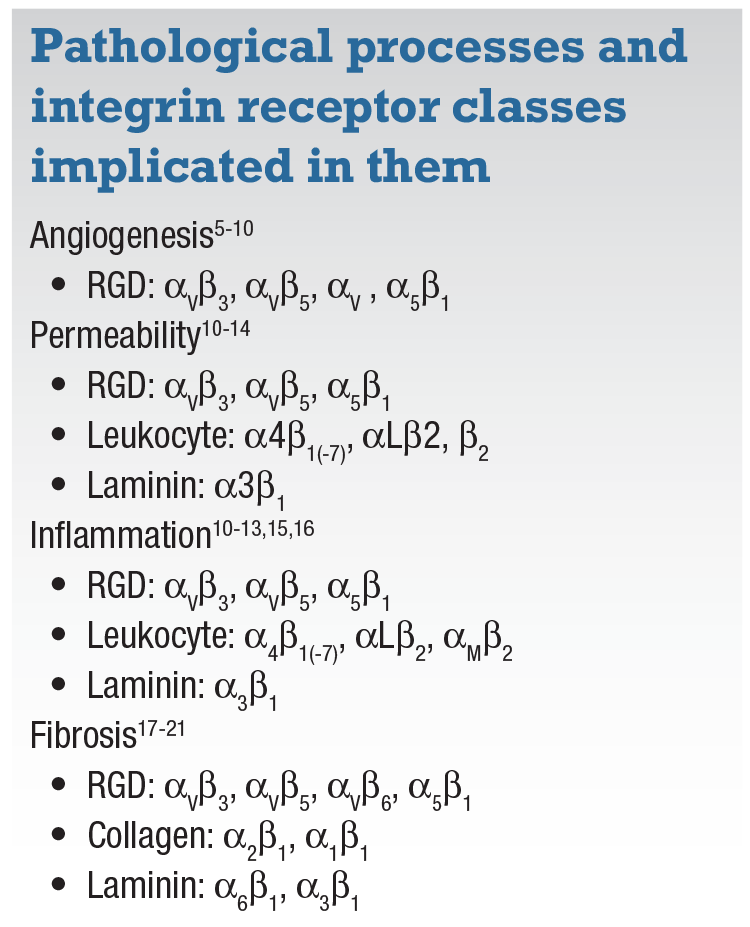
They each play a different role in the four primary pathologic processes: angiogenesis;5-10 permeability;10-14 inflammation;10-13,15,16 and fibrosis17-21 (Table, page 30). Multiple RGD integrin receptors have been implicated in the disease processes of wet AMD and DR. For example, targeting specific subunits of RGD, collagen and integrin receptors would effectively target fibrosis, whereas angiogenesis is a function of specific RGD receptors.
 |
Risuteganib
Risuteganib (RSG) is a synthetic, RGD-class peptide that targets the retinal pigment epithelium and the outer retina. It has a molecular weight of 750 Dalton and a very long half-life of 21 days. It is designed to regulate three integrin isoforms identified in the pathological process:
- αVβ5, which signals angiopoietin-2 (Ang-2)-induced astrocyte apoptosis, contributing to vascular leakage in DR;
- α5β1, a contributor to the angiogenesis pathway that’s different from vascular endothelial growth factor-mediated angiogenesis; and
- αMβ2, an isoform involved in inflammatory response.
 |
Julie Kornfield, PhD, at the California Institute of Technology, and Peter Campochiaro, MD, at Johns Hopkins University, have reported that RSG downregulates genes associated with five angiogenesis-
related biological processes:
- Integrin-mediated signaling pathway.
- Tube development.
- Extracellular matrix organization.
- Circulatory system development.
- Response to stimulus.
RSG has also been shown to reduce neovascularization in the ischemic retinopathy model. Additionally, Dr. Campochiaro and colleagues have reported that RSG reduced the total area of neovascularization by approximately 87 percent, vs. approximately a 50-percent reduction for aflibercept (Eylea, Regeneron).22
RSG has also been shown to downregulate gene expression in human immune cells, which have shown a proven immune and inflammatory response, and a response to stimuli. (These integrin receptors become activated only when an insult occurs; otherwise, they don’t have any significant response.)
Glenn Jaffe, MD, and colleagues at Duke Eye Center reported on the protective effects of RSG in cultured human RPE cells.23 Relative growth in cells treated with RSG was 34 percent vs. 18 percent in non-RSG cells; cell viability in terms of mitochondrial membrane potential was 79 percent vs. 46 percent; and 74 percent of cells with RSG exhibited cell viability vs. 42 percent of non-RSG cells.
 |
Findings of DEL MAR trial
The DEL MAR Phase II trial has demonstrated the efficacy and safety of RSG in patients with DME. The study reported that previously treated patients on RSG had more robust visual gains compared to treatment-naïve patients, and that a regimen of three monthly injections of RSG was non-inferior to monthly bevacizumab (Avastin, Genentech/Roche).
Stage one of the Phase II DEL MAR study evaluated patients on RSG 1 and 3 mg and bevacizumab 1.25 mg monthly for three months. The RSG 1-mg group had better overall results than the other RSG dosing groups. At 20 weeks, the previously treated patients on RSG 1 mg had a more robust response than the treatment-naïve patients, with a mean gain of 20 Early Treatment Diabetic Retinopathy Study letters vs. 11.
In DEL MAR stage 2, overall mean change in ETDRS letters at 20 weeks was similar between the 1.25-mg bevacizu-
mab-only group and the 1.25-mg bevacizumab/1-mg RSG combination group: 6.7 for the former and 7.1 for the latter.
This stage of the study also showed that the sequence of the drugs was important. Patients first given bevacizumab and then given three injections of RSG had a more profound response than those given the drugs simultaneously. This applied in both the previously treated (24-letter gain) and the treatment-naïve (17-letter gain) groups, with no improvement in the simultaneously dosed patients. The Phase II study of RSG for dry AMD is ongoing.
 |
Topical SF0166
SF0166 (SciFluor Life Sciences) is a selective, small-molecule inhibitor applied topically. It inhibits the RGD integrin receptor αVβ3, a non-fluorinated factor in angiogenesis. The fluorinated molecule structure of SF0166 enables it to penetrate the sclera and allows broad distribution of the drug. SF0166 has been found in high concentrations in the sclera and retina-choroid plexus for at least 12 hours after administration.24
Preclinical studies have shown SF0166 reduces VEGF-induced vascular leakage comparably to bevacizumab.25 Results of a Phase I/II trial in patients with DME have so far shown no corneal toxicity or other drug-related serious adverse events. The study has also found measurable changes in central subfield thickness (CST) after treatment in both the 2.5% and 5% SF0166 treatment groups. At the end of treatment on day 28, 10 of 35 subjects showed decreases in CST, but at the end of the study on day 56, 19 of 38 subjects showed a decrease in CST. This illustrates a trend toward increased anatomic response from day 28 to day 56 of treatment. Of note: The trial has found no overall change in visual acuity and no correlation between CST and BCVA changes.
The safety profile coupled with evidence of biological activity warrants further study of SF0166 in Phase II trials investigating expansion of the dose range, as well as treatment and follow-up periods.
THR-687
THR-687 (Oxurion) is a highly potent, small-molecule pan-RGD integrin antagonist. In preclinical study, it has been shown to inhibit endothelial cell migration, which is involved in angiogenesis.26 Extensive toxicology and safety pharmacology studies indicate a good safety profile for this drug. It has broad therapeutic potential for treating DR with or without DME, wet AMD and anti-VEGF nonresponders. The first patient in a Phase I trial for treatment of DME has been enrolled. RS
REFERENCES
1. Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007; 213: 565-573 .
2. Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697-715.
3. Gonzalez-Salinas R, Hernández-Zimbrón LF, Gulias-Cañizo R, et al. Current anti-integrin therapy for ocular disease. Semin Ophthalmol. 2018;33:634-642.
4. Goswami LN, Ma L, Cai Q,et al. cRGD peptide-conjugated icosahedral closo-B12(2-) core carrying multiple Gd3+-DOTA chelates for α(v)β3 integrin-targeted tumor imaging (MRI). Inorg Chem. 2013 18;52:1701-1709.
5. Friedlander M, Theesfeld CL, Sugita M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93:9764-9769.
6. Umeda N, Kachi S, Akiyama H, et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820-1828.
7. Wilkinson-Berka JL, Jones D, Taylor G, et al. SB-267268, a nonpeptidic antagonist of alpha(v)beta3 and alpha(v)beta5 integrins, reduces angiogenesis and VEGF expression in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006 Apr;47:1600-1605.
8. Fu Y, Ponce ML, Thill M, Yuan P, Wang NS, Csaky KG. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest Ophthalmol Vis Sci. 2007;48:5184-5190.
9. Hammes HP, Weiss A, Führer D, Krämer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996;39:251-255
10. Santulli RJ, Kinney WA, Ghosh S, et al. Studies with an orally bioavailable alpha V integrin antagonist in animal models of ocular vasculopathy: retinal neovascularization in mice and retinal vascular permeability in diabetic rats. J Pharmacol Exp Ther. 2008;324:894-901
11. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450-2.
12. Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:4898-4904.
13. Rao Malla R, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS. Knockdown of cathepsin B and uPAR inhibits CD151 and α3β1 integrin-mediated cell adhesion and invasion in glioma. Mol Carcinog. 2013;52:777-790.
14. Park SW, Yun JH, Kim JH, Kim KW, Cho CH, Kim JH. Angiopoietin 2 induces pericyte apoptosis via α3β1 integrin signaling in diabetic retinopathy. Diabetes. 2014;63:3057-3068.
15. Kanda A, Noda K, Oike Y, Ishida S. Angiopoietin-like protein 2 mediates endotoxin-induced acute inflammation in the eye. Lab Invest. 2012;92:1553-163.
16. Hirasawa M, Takubo K, Osada H, et al. Angiopoietin-like protein 2 is a multistep regulator of inflammatory neovascularization in a murine model of age-related macular degeneration. J Biol Chem. 2016;291:7373-7785.
17. Robbins SG, Brem RB, Wilson DJ, er al. Immunolocalization of integrins in proliferative retinal membranes. Invest Ophthalmol Vis Sci. 1994;35:3475-3485.
18. Ning A, Cui J, Maberley D, Ma P, Matsubara J. Expression of integrins in human proliferative diabetic retinopathy membranes. Can J Ophthalmol. 2008;43:683-688.
19. Zahn G, Volk K, Lewis GP, et al. Assessment of the integrin alpha5beta1 antagonist JSM6427 in proliferative vitreoretinopathy using in vitro assays and a rabbit model of retinal detachment. Invest Ophthalmol Vis Sci. 2010;51:1028-35.
20. Blanco-Mezquita JT, Hutcheon AE, Stepp MA, Zieske JD. αVβ6 integrin promotes corneal wound healing. Invest Ophthalmol Vis Sci. 2011;52:8505-8513.
21. Wang S, Park JK, Duh EJ. Novel targets against retinal angiogenesis in diabetic retinopathy. Curr Diab Rep. 2012;12:355-363.
22. Silva RLE, Kanan Y, Campochiaro PA, et al. Tyrosine kinase blocking collagen IV-derived peptide suppresses ocular neovascularization and vascular leakage. Sci Transl Med. 2017;9. pii: eaai8030
23. Yang P, Baciu P, Jaffe GJ, et al. Retinal pigment epithelial cell death by the alternative complement cascade: Role of membrane regulatory proteins, calcium, PKC, and oxidative stress. Invest Ophthalmol Vis Sci. 2014;55:3012-3021.
24. Press release SciFluor Life Sciences. SciFluor announces positive results of Phase I/II study of SF0166 topical ophthalmic solution in diabetic macular edema patients. Cambridge, MA: September 28, 2017.
25. SciFluor Life Sciences. Data on file.
26. Shao Z. Friedlander M, Hurst CG, et al. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One. 2013;8:e69552.



