 |
 |
Closure of a macular hole is accomplished by a coordinated wound healing response executed by activated neighboring glial cells. Proliferation and migration of activated glia are followed by their contraction, which leads to macular hole closure by reapposition of the edges of the retinal defect.1-3
It is well documented that small macular holes have the capacity of self-closure without surgical intervention, and that small macular holes that don’t heal spontaneously do well with the standard surgical approach of vitrectomy, internal limiting membrane peeling and gas endotamponade. These interventions augment the gliotic response needed for macular hole closure.4-6
For small macular holes, glial migration and macular hole closure occur relatively easily, as the defect is generally small and relatively acute. On average, the success rate of macular hole closure with surgery is around 90 percent.7 However, this closure rate has an inverse relationship with the size, chronicity and refractory status of the macular hole being operated on. Large, chronic or recurrent holes generally demonstrate lower closure rates.7, 8
Role of guided wound healing
In these more difficult cases, guided wound healing is generally the preferred approach. Surgical closure approaches to large or chronic holes entail the use of biologic scaffolds that allow for glial cell migration to traverse the large retinal defect in a controlled manner.
Here we report on the early experience with epiretinal human amniotic membrane (hAM) grafting in chronic, refractory macular holes. Human amniotic membrane appears to possess the ideal properties to correct the aberrant wound healing of a macular hole. It provides an anti-inflammatory, neurotrophic environment with a structural biologic scaffold for glial cell migration to facilitate wound closure.
 |
Alternative to biological scaffolds
Several biologic scaffolds have received attention in recently described surgical methods to address large, myopic or chronic macular holes, including the inverted ILM flap, autologous ILM transplantation, anterior lens capsule flaps as well as the use of neurosensory retinal autologous transplantation of a neurosensory retinal flap for refractory holes.9-12
An alternative is the use of hAM, which has proven successful as a scaffold for healing the cornea and conjunctiva.13 Only 20 to 50 μm thick and derived from the innermost layer of the placenta, hAM appears to be an ideal scaffold for wound healing, particularly within macular holes.
Not only does hAM provide a scaffold for controlled and guided gliosis, but it lacks immunogenicity, has anti-inflammatory and anti-angiogenic properties, is inert, and has been shown to support retinal pigment epithelium growth in vitro.13-15 Because of its anti-inflammatory and pro-healing tendencies, human amniotic membrane may in fact provide a biologic structural scaffold superior to even autologous tissues such as neurosensory retinal flaps.
 |
Higher levels of protein expression
We conducted a study to compare the glial activation and inflammatory response induced by covering macular holes with autologous human retina explants or hAM.6 We used 19 freshly harvested human cadaveric eyes, into which 1-mm circular holes were made.
These holes were then covered in an epiretinal fashion with 3-mm grafts consisting of hAM or autologous neurosensory retina (ANR), or were left uncovered for three days. We compared the gliotic reaction these graft states induced using a variety of methods aimed at detecting glial cell markers, including bone morphogenetic protein 7 (BMP7), glial fibrillary acidic protein (GFAP) and vimentin, within the explanted retina.
Western-blot techniques revealed that hAM expressed higher levels of BMP7, GFAP and vimentin compared to ANR, suggesting that the gliotic signaling response and recruitment with hAM is comparatively more robust at the translational level. One other marker we tested was
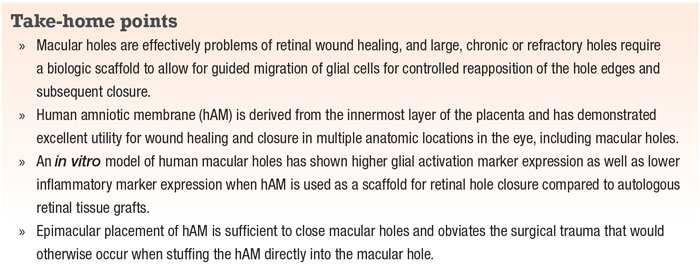
expression of the SERPINA3 gene, which encodes for a serine protease inhibitor expressed during inflammation and as an acute phase protein.6 Using protein quantitation and immunohistochemical techniques, we found that this gene was more highly expressed in retinal holes covered with ANR compared to hAM, suggesting that hAM exhibited anti-inflammatory and likely pro-wound healing properties that bypassed the need for endogenous expression of acute phase proteins within the retina (Figure 1, page 19).
Taken together, these experiments show that hAM is in fact a more ideal biologic scaffold that provides the underlying retina with neurotrophic and anti-inflammatory signals that promote hole closure more robustly than autologous tissues.
 |
Emerging evidence for hAM
Stanislao Rizzo, MD, and colleagues at the University of Florence recently popularized the use of hAM.16 They demonstrated the surgical technique of employing transplantation of human amniotic membrane into the subretinal space underneath recurrent macular holes with gas endotamponade resulted in good anatomic and visual outcome.
 |
Other reports have used a similar technique of placement of hAM directly into the hole, with reports of postoperative parafoveal atrophy in 40 percent of patients.17,18 However, other groups have shown that placement of the amniotic membrane in an epimacular fashion under gas can also result in good outcome, with reattachment of retina as well as hole closure and improvement in visual acuity.19
From a mechanistic perspective, an epimacular placement should work just as effectively with hAM, because all that’s needed is a scaffold to allow for guided glial cell migration. This type of placement is furthermore less traumatic to the macula because it bypasses the necessary trauma to the edges of the hole when stuffing the hAM into the defect.
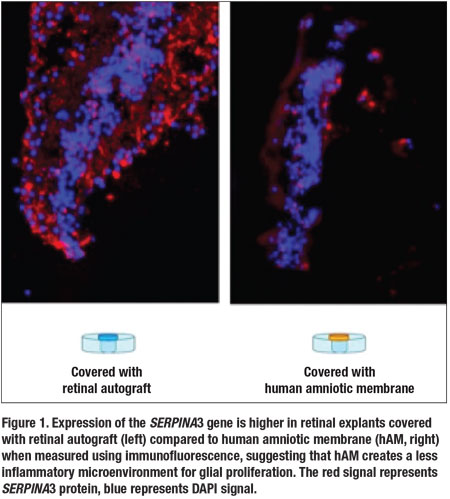
In fact, using immunohistochemistry and our in vitro system described previously, we were able to demonstrate successful glial migration and subsequent reapposition of retinal wound edges through epiretinal coverage using hAM scaffold (Figure 2). This suggests that all that glial cells need to close the hole is a scaffold to guide migration over the hole rather than direct contact of the hAM with the walls of the hole.
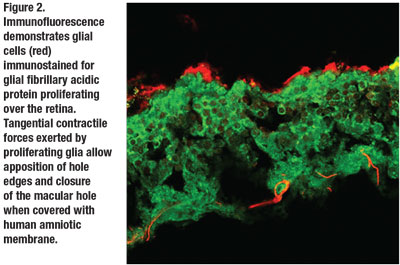
Moreover, we devised a technique to address these macular hole-associated retinal detachments with staphyloma. This involves affixing a large amniotic membrane graft over the area of the macular hole and applying 5,000 centistoke silicone oil for endotamponade (Figure 3 and video).
Alternatively, stabilizing the hAM graft using a tissue glue made with the patient’s own plasma can prevent its dislodging should a gas tamponade be preferred at the end of the surgery. In our experience, this technique has resulted in excellent postoperative outcomes.
Human amniotic membrane appears to display the ideal properties to correct the aberrant wound healing of a macular hole. It provides an anti-inflammatory, neurotrophic environment, while also providing a structural biologic scaffold for glial cell migration to facilitate wound closure.
Bottom line
Higher expression of glial cell activation markers, as well as decreased expression of anti-inflammatory markers in wounded retina covered with hAM suggests that for chronic, large and refractory holes, hAM may provide the best option for the desired surgical outcome.
We also recommend the use of hAM in an epimacular fashion rather than subretinal placement, given that glial cells can migrate across the hAM scaffold in an epimacular fashion and achieve hole closure effectively by bringing the hole edges together. This reduces surgical trauma to the photoreceptors in the macula, preserving the best possible visual acuity. RS
Watch as Dr. Tezel demonstrates the technique for using human amniotic membrane to repair macular hole surgery-associated retinal detachments with staphyloma.
REFERENCES
1. Rosa RH, Jr., Glaser BM, de la Cruz Z, Green WR. Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor-beta 2, and gas tamponade. Am J Ophthalmol. 1996;122:853-863.
2. Bringmann A, Duncker T, Jochmann C, Barth T, Duncker GIW, Wiedemann P. Spontaneous closure of small full-thickness macular holes: Presumed role of Muller cells. Acta Ophthalmologica. 2020;98:e447-e456.
3. Schubert HD, Kuang K, Kang F, Head MW, Fischbarg J. Macular holes: migratory gaps and vitreous as obstacles to glial closure. Graefes Arch Clin Exp Ophthalmol. 1997;235:523-529.
4. Liang X, Liu W. Characteristics and Risk factors for spontaneous closure of idiopathic full-thickness macular hole. J Ophthalmol. 2019;2019:4793764.
5. Parravano M, Giansanti F, Eandi CM, Yap YC, Rizzo S, Virgili G. Vitrectomy for idiopathic macular hole. Cochrane Database Syst Rev. 2015:CD009080.
6. Tezel TH, Karahan E, Hondur A, Zeng Q. Covering macular hole with human amniotic membrane promotes more pronounced wound healing response compared to retinal autografts. Paper presented at American Society of Retina Specialists 37th annual scientific meeting; Chicago, IL; July 28, 2019.
7. la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002;80:579-587.
8. Kobayashi H, Kobayashi K. Correlation of quantitative three-dimensional measurements of macular hole size with visual acuity after vitrectomy. Graefes Arch Clin Exp Ophthalmol. 1999;237:283-288.
9. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018-2025.
10. Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36:163-170.
11. Grewal DS, Fine HF, Mahmoud TH. Management of challenging macular holes: current concepts and new surgical techniques. Ophthalmic Sur Lasers Imaging Retina. 2016;47:508-513.
12. Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134:229-230.
13. Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014;4:111-121.
14. Capeans C, Pineiro A, Pardo M, et al. Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand. 2003;81:271-277.
15. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349:447-458.
16. Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39 Suppl 1:S95-S103.
17. Abouhussein MA, Elbaha SM, Aboushousha M. Human amniotic membrane plug for macular holes coexisting with rhegmatogenous retinal detachment. Clin Ophthalmol. 2020;14:2411-2416.
18. Tsai DC, Huang YH, Chen SJ. Parafoveal atrophy after human amniotic membrane graft for macular hole in patients with high myopia. Br J Ophthalmol. Published online July 31, 2020.
19. Moharram HM, Moustafa MT, Mortada HA, Abdelkader MF. Use of epimacular amniotic membrane graft in cases of recurrent retinal detachment due to failure of myopic macular hole closure. Ophthalmic Sur Lasers Imaging Retina. 2020;51:101-108.



