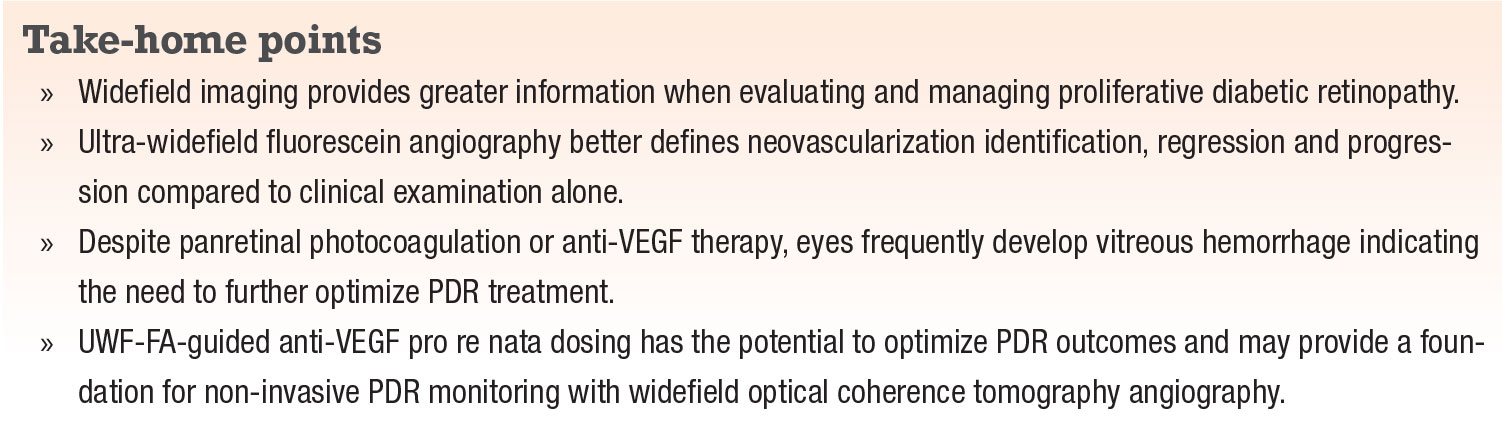
 |
 |
The consequences of proliferative diabetic retinopathy, if uncontrolled, are well known: severe vision loss with vitreous hemorrhage and tractional retinal detachment.1 While pan-retinal photocoagulation has remained the standard therapy, DRCR Retina Network Protocol S and CLARITY trial results established intravitreal injections with anti-VEGF drugs as an alternative therapy for PDR eyes that don’t require vitrectomy.
Anti-VEGF agents have been shown to produce superior or equivalent visual outcomes while minimizing visual field loss and proliferative consequences compared with PRP.2-4 While anti-VEGF agents offer many benefits, therapy is more expensive, the treatment burden is higher and follow-up and monitoring more frequent than PRP alone. Patient non-compliance with follow-up has remained an issue.3,5
Therefore, retina specialists often opt to administer PRP alone or in combination with anti-VEGF for PDR because PRP is thought to be more reliable in this population.6
Despite the favorable and equivalent five-year outcomes observed for both PRP and ranibizumab-treated eyes in DRCR Protocol S, vitreous hemorrhage occurs in almost 50 percent of eyes receiving either therapy.3 The high incidence of proliferative complications indicates a need to evaluate how to further optimize PDR treatment.
Ultra-widefield imaging
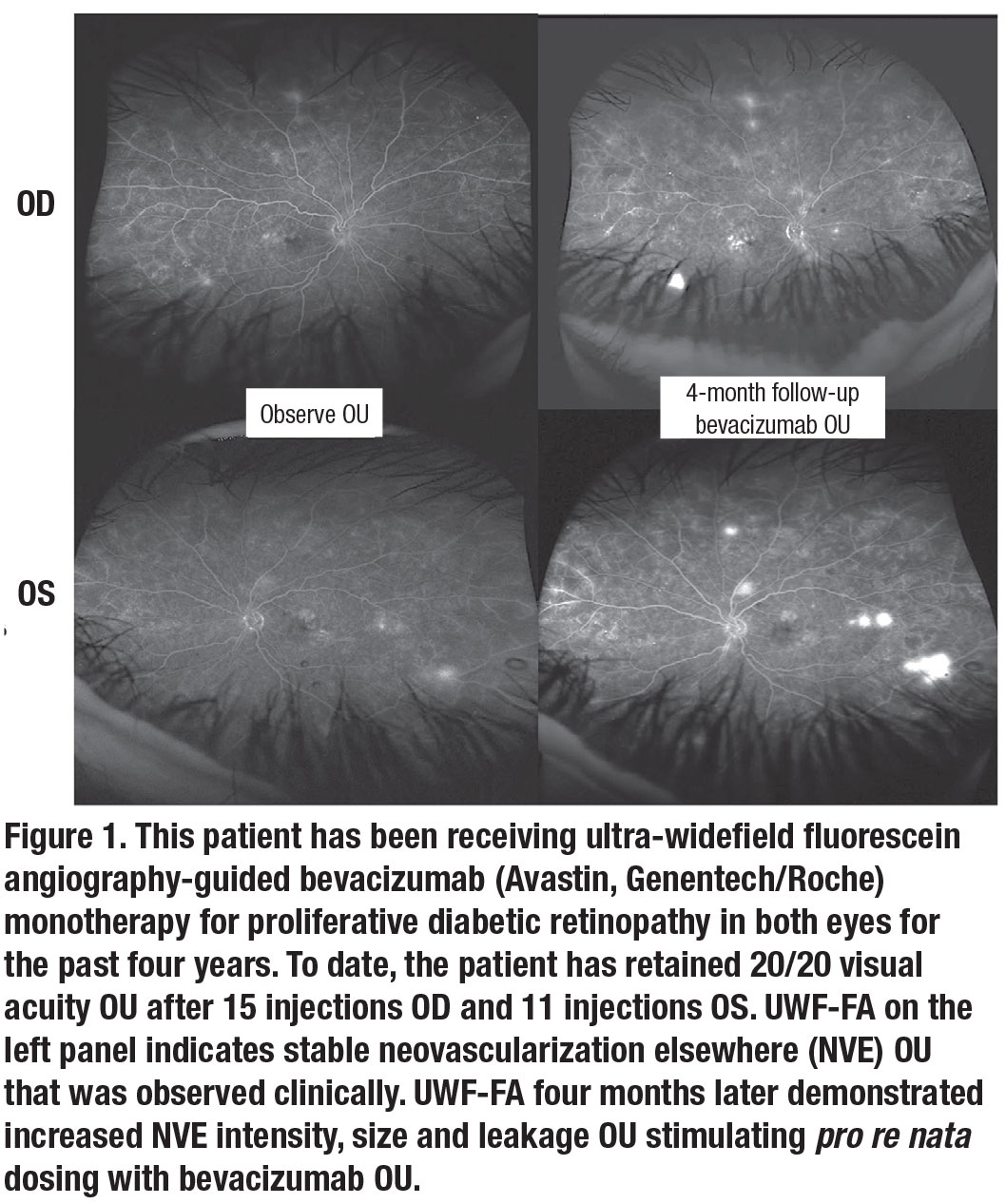 |
Since 1991, diabetic retinopathy severity and progression have been graded based on the modified Airlie House classification using seven-standard field fundus (7SF) photography established in the Early Treatment Diabetic Retinopathy Study, which captures about 35 percent of the retinal area.7-9 Imaging the peripheral retina can identify more pathology and provide further guidance in managing PDR.10
Ultra-widefield imaging, capturing up to 82 percent of retinal area, provides more information.10,11 With greater adoption of widefield imaging in clinical practice, UWF imaging was recently defined as a single image centered on the fovea capturing retinal anatomy beyond the posterior pole and anterior to the vortex veins in all four quadrants.12
The multicenter DRCR Protocol AA study along with other single-center studies have shown agreement in assessing DR severity on EDTRS images and UWF fundus photography.13-16
Retinopathy peripheral to the EDTRS fields is identified in up to 40 percent of eyes on UWF imaging, resulting in increased DR severity in 9 to 15 percent of eyes. Eyes with peripheral retinopathy have also been shown to have higher rates of DR progression compared to eyes without peripheral retinopathy.10,11,13-23
 |
For years, the American Academy of Ophthalmology preferred practice patterns didn’t recommend FA as a necessity in the diagnosis and management of PDR. With the incorporation of widefield FA as a supplement to fundus photography and clinical examination, it has emerged as a valuable tool.8,24
UWF-FA obtains images with 3.2 times more retinal area than the 7SF images, revealing nonperfusion, ischemia, vascular leakage and neovascularization not evident with fundus photography or clinical examination. These lesions have clinically significant implications on PDR management.6,11,22,23,25-27 Area of retinal nonperfusion, often imaged in the mid-peripheral retina, is predictive of developing or finding PDR and neovascularization.28
UWF-FA Guided Anti-VEGF Therapy
PDR eyes treated with anti-VEGF agents have shown retinal neovascularization regression and reperfusion on both widefield and standard-field FA.29-33 Our clinical experience indicates that UWF-FA identifies neovascularization regression and progression better than clinical examination alone. So, when electing anti-VEGF monotherapy for PDR, we’ve found using UWF-FA beneficial (Figure 1). Our experience in the care of PDR eyes led us to include this approach within our clinical trial (LASERLESS Trial) evaluating endolaserless (omitting PRP endolaser) vitrectomy for PDR eyes with vitreous hemorrhage. We utilized UWF-FA-guided aflibercept (Eylea, Regeneron Pharmaceuticals) PRN dosing (in addition to mandatory postoperative aflibercept) in PDR eyes after endolaserless vitrectomy.
We previously reported results for 31 LASERLESS trial eyes.34-37 Four-month results demonstrated quick visual acuity (VA) gains with an improvement of 38 to 72 (20/40) letters.35 One-year results indicated safety, moderate-term durability and significant VA improvement.36
Additionally, neovascularization was absent on UWF-FA in 70 percent and 27 percent of q8- and q16-week eyes, respectively. Our two-year results indicate that persistent and frequent postoperative anti-VEGF therapy is necessary to optimize visual outcomes and reduce complications.37
We also evaluated using UWF-FA to guide pro re nata aflibercept monotherapy in 17 PRP-naïve PDR eyes not requiring vitrectomy (Figures 2 and 3). Through one year, we observed excellent safety and a 4-letter VA gain. We also observed an absence of neovascularization on UWF-FA in 41 percent of eyes at four weeks after the first aflibercept injection. Progression after regression of neovascularization occurred as only 24 percent of eyes demonstrated absence of neovascularization at one year despite an average of 5.7 injections administered.38
Is PRP more durable?
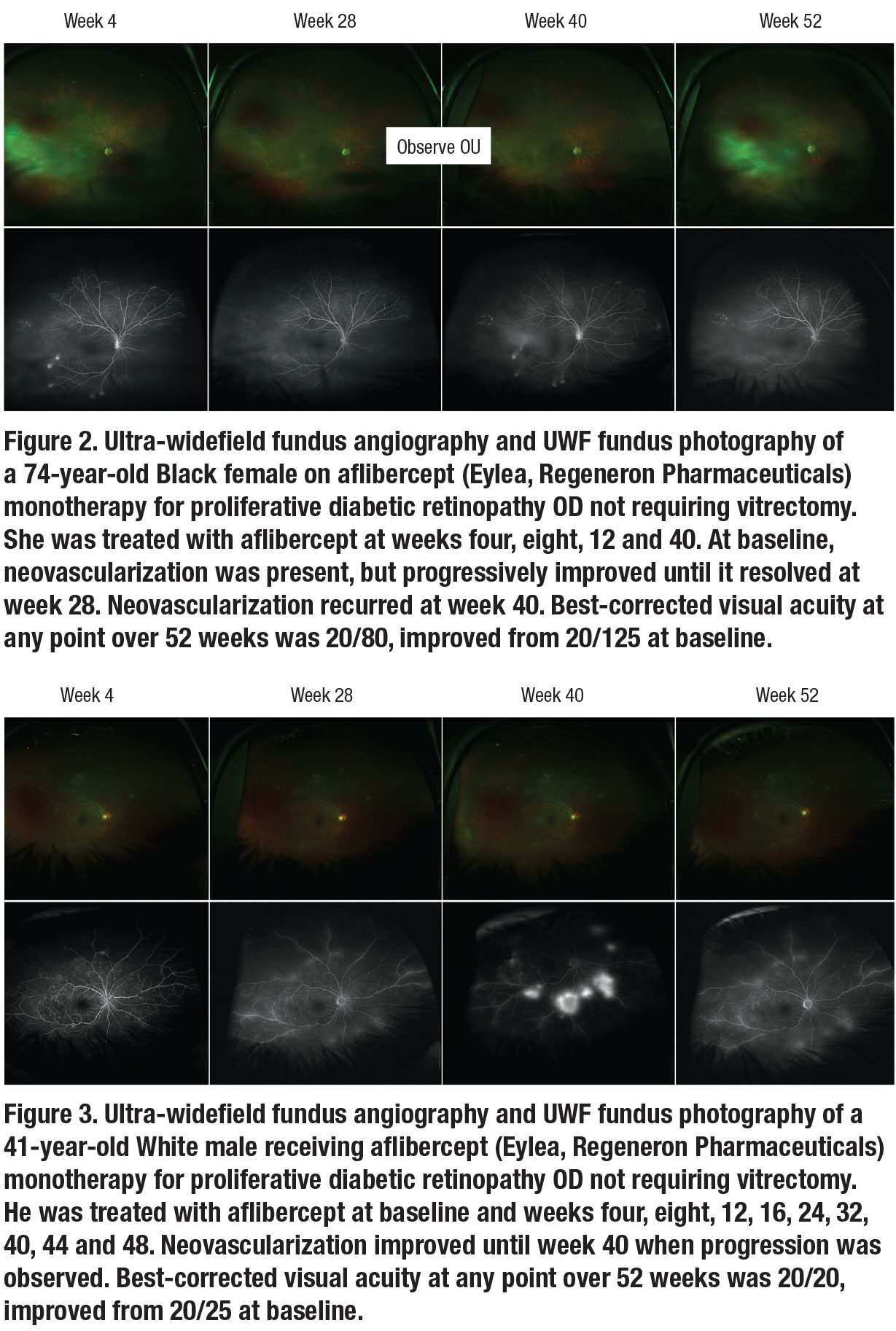 |
While PRP is commonly believed to be more durable in treating PDR than anti-VEGF agents, it hasn’t been definitively confirmed in major PDR trials. In the CLARITY study, 65 and 6 percent of eyes required additional PRP and vitrectomy, respectively, through one year.4 In the PROTEUS study, 44 percent of eyes receiving PRP and ranibizumab showed complete neovascularization regression compared to 25 percent of eyes receiving PRP alone.39
In many large PDR trials, neovascularization status was monitored by clinical examination alone, which may have resulted in missed neovascularization progression. Without the use of widefield FA, we may be undertreating PDR, resulting in significant vitreous hemorrhage rates. Thus, UWF-FA guided anti-VEGF PRN dosing has the potential to reduce proliferative complications and vitreous hemorrhage rates in PDR eyes even after PRP.
Widefield OCTA
Optical coherence tomography angiography, a newer imaging method that allows for three-dimensional visualization of the retinal microvasculature, has been reported to successfully depict DR lesions, even revealing vascular abnormalities in people with diabetes with a normal fundus on ophthalmoscopy.24 It offers advantages over FA, including the ability to view neovascularization and determine its location as preretinal or intraretinal.40
OCTA can also detect diabetic macular ischemia as well as FA.41,42 Recent studies have demonstrated that widefield OCTA may be an appropriate imaging modality for managing PDR, providing information about neovascularization comparable to UWF-FA.43 Figure 4 compares widefield OCTA with standard field OCTA.
Widefield OCTA has been proposed to be the only imaging modality required to monitor neovascularization status in PDR after PRP.40 It’s important to note, however, that while FA can show decreased neovascularization leakage after PRP or anti-VEGF, OCTA can’t detect this, thus potentially limiting its ability to assess neovascularization progression or regression.44 However, as OCTA detects nonperfusion better than FA, the need to further evaluate its role in PDR management exists.26,45
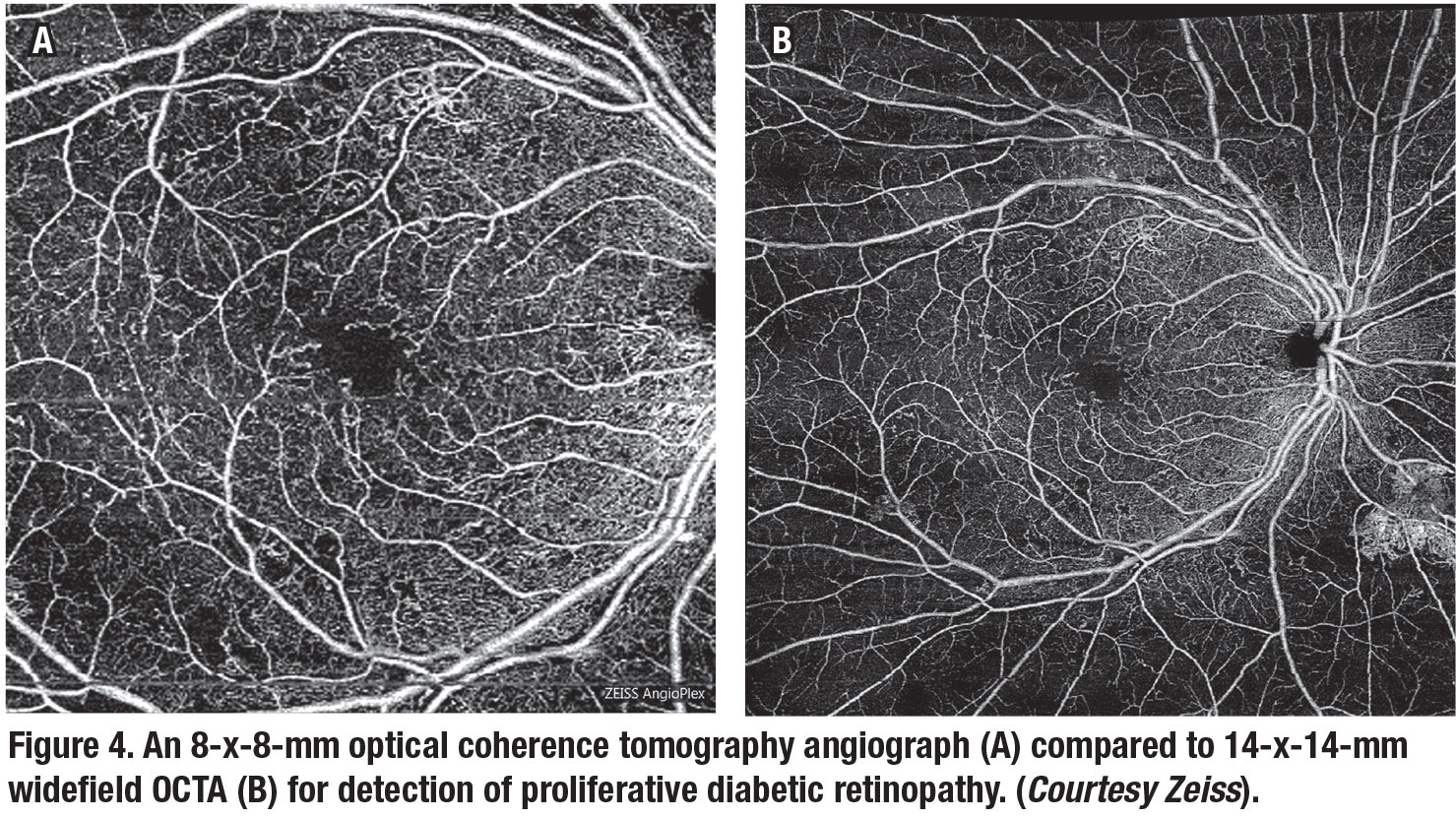 |
 |
Future study focus
Even though UWF-FA is frequently used in clinical practice, limited data exists evaluating UWF-FA-guided PRN anti-VEGF dosing. However, as UWF imaging has been shown to detect peripheral retinopathy and neovascularization not easily observed on clinical exam and fundus photography, we believe that wide-field angiographic monitoring is a useful tool to monitor proliferative activity and optimize anti-VEGF therapy needs, thus reducing proliferative consequences.
Using UWF imaging in PDR eyes is especially important because neovascularization assessment on fundus exam can be confounded by a lack of patient cooperation, phakic status, and profound retinal ischemia and flat neovascularization that are difficult to assess on exam. While our widefield FA monitoring approach is limited by absence of control groups, it’s analogous to early nAMD trials (PrONTO), which based anti-VEGF treatment on anatomical changes observed on OCT.46
Bottom line
UWF-FA guided anti-VEGF PRN dosing has the potential to optimize PDR outcomes and may provide a foundation for non-invasive PDR monitoring with widefield OCTA, especially as continued use and advancements in wide-angle and widefield montage OCTA emerge.24,26,40-45
We also hope that our widefield FA-guided findings stimulate further evaluation on monitoring PDR activity with UWF-FA and of various dosing regimens for PDR. DRCR Retina Network Protocol AA results will provide additional important information on widefield imaging in DR. RS
REFERENCES
 |
1. Liu TYA, Arevalo JF. Wide-field imaging in proliferative diabetic retinopathy. Int J Retina Vitreous. 2019;5(Suppl 1):20.
2. Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314:2137-2146.
3. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA Ophthalmol. 2018;136:1138-1148.
4. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193-2203.
5. Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-vegf injections. Ophthalmology. 2018;125:1386-1392.
6. Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25:27-33.
7. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):823-833.
8. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786-806.
9. Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682.
10. Ashraf M, Shokrollahi S, Salongcay RP, Aiello LP, Silva PS. Diabetic retinopathy and ultrawide field imaging. Semin Ophthalmol. 2020;35:56-65.
11. Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785-791.
12. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the international widefield imaging study group. Ophthalmol Retina. 2019;3:843-849.
13. Aiello LP, Odia I, Glassman AR, et al. Comparison of early treatment diabetic retinopathy study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65-73.
14. Kernt M, Hadi I, Pinter F, et al. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care. 2012;35:2459-2463.
15. Rasmussen ML, Broe R, Frydkjaer-Olsen U, et al. Comparison between Early Treatment Diabetic Retinopathy Study 7-field retinal photos and non-mydriatic, mydriatic and mydriatic steered widefield scanning laser ophthalmoscopy for assessment of diabetic retinopathy. J Diabetes Complications. 2015;29:99-104.
16. Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154:549-559.e542.
17. Neubauer AS, Kernt M, Haritoglou C, Priglinger SG, Kampik A, Ulbig MW. Nonmydriatic screening for diabetic retinopathy by ultra-widefield scanning laser ophthalmoscopy (Optomap). Graefes Arch Clin Exp Ophthalmol. 2008;246:229-235.
18. Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. Clin Ophthalmol. 2015;9:527-531.
19. Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949-956.
20. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587-2595.
21. Silva PS, Cavallerano JD, Tolls D, et al. Potential efficiency benefits of nonmydriatic ultrawide field retinal imaging in an ocular telehealth diabetic retinopathy program. Diabetes Care. 2014;37:50-55.
22. Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465-2472.
23. Talks SJ, Manjunath V, Steel DH, Peto T, Taylor R. New vessels detected on wide-field imaging compared to two-field and seven-field imaging: implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 2015;99:1606-1609.
24. Yang JY, Wang Q, Yan YN, et al. Microvascular retinal changes in pre-clinical diabetic retinopathy as detected by optical coherence tomographic angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258:513-520.
25. Sim DA, Keane PA, Rajendram R, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158:144-153.e141.
26. Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126:1685-1694.
27. Wessel MM, Nair N, Aaker GD, Ehrlich JR, D'Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694-698.
28. Baxter SL, Ashir A, Nguyen BJ, Nudleman E. Quantification of retinal nonperfusion associated with posterior segment neovascularization in diabetic retinopathy using ultra-widefield fluorescein angiography. Ophthalmic Surg Lasers Imaging Retina. 2019;50:86-92.
29. Adamis AP, Altaweel M, Bressler NM, et al. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113:23-28.
30. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1691-1615.
31. Chandra S, Sheth J, Anantharaman G, Gopalakrishnan M. Ranibizumab-induced retinal reperfusion and regression of neovascularization in diabetic retinopathy: An angiographic illustration. Am J Ophthalmol Case Rep. 2018;9:41-44.
32. González VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93:1474-1478.
33. Levin AM, Rusu I, Orlin A, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol. 2017;11:193-200.
34. D’Amico DJ. From the Editor-in-Chief. J VitreoRetinal Dis. 2018;2:125-126.
35. Marcus DM, Singh H, Starnes DC, et al. Endolaserless vitrectomy with intravitreal aflibercept injection for proliferative diabetic retinopathy-related vitreous hemorrhage (LASER LESS TRIAL). J VitreoRetinal Dis. 2018;2:127-137.
36. Marcus DM. Endolaserless vitrectomy with intravitreal aflibercept injection (IAI) for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage: 1 year results (LASER LESS TRIAL). Paper persented at American Society of Retina Specialists annual meeting; July 20-25, 2018; Vancouver, BC, Canada.
37. Levy R, Marcus DM, Starnes D, Pooley P. Complications, compliance and 2 year outcomes after endolaserless vitrectomy with aflibercept monotherapy for pdr-related vitreous hemorrhage. Invest Ophthalmol Vis Sci. 2020;61:1380.
38. Marcus DM, Taylor C, Starnes D, et al. Ultra wide-field fluorescein angiographic-guided aflibercept (WFFAGA) monotherapy for proliferative diabetic retinopathy (PDR). J Clin Ophthalmol. 2019;3:166-173.
39. Figueira J, Fletcher E, Massin P, et al. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS Study). Ophthalmology. 2018;125:691-700.
40. Russell JF, Shi Y, Hinkle JW, et al. Longitudinal wide-field swept-source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Retina. 2019;3:350-361.
41. Garcia JM, Lima TT, Louzada RN, Rassi AT, Isaac DL, Avila M. Diabetic macular ischemia diagnosis: comparison between optical coherence tomography angiography and fluorescein angiography. J Ophthalmol. 2016;2016:3989310.
42. Bradley PD, Sim DA, Keane PA, et al. The evaluation of diabetic macular ischemia using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:626-631.
43. Russell JF, Flynn HW, Jr., Sridhar J, et al. Distribution of diabetic neovascularization on ultra-widefield fluorescein angiography and on simulated widefield OCT angiography. Am J Ophthalmol. 2019;207:110-120.
44. Sawada O, Ichiyama Y, Obata S, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:1275-1280.
45. Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am J Ophthalmol. 2015;160:35-44.e31.
46. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43-58.e41.



