Take-home points
|
 |
|
Bios Dr. Lee is a vitreoretinal surgeon at Dr. Everett Chalmers Hospital, Fredericton, New Brunswick, Canada. |
Several advances in our surgical techniques have emerged over the past century, and we are fortunate to have multiple treatment options for rhegmatogenous retinal detachment, including pneumatic retinopexy, scleral buckle and pars plana vitrectomy.
Despite the availability of these options, the debate over the best approach for RRD repair has been ongoing.1–3 This is related in part to limited randomized trials comparing various treatment options for RRD.
However, advancements in retinal imaging technology over the years have allowed us to gather more data and to better understand the process of retinal detachment and reattachment, which may potentially assist retinal surgeons in making treatment decisions and planning future studies involving a variety of anatomic imaging biomarkers.
A change in perspective on RRD repair
Advancements in microsurgical instrumentation over the past few decades have improved the single-surgery success rates of RRD repair. However, despite successful anatomic reattachment, patients may still experience suboptimal functional outcomes and complain of reduced visual acuity, metamorphopsia or aniseikonia.4–9 This has led to a pivotal change in how we view a successful RRD repair from the traditional anatomic reattachment to achieving structural integrity of the retinal reattachment.
Several authors have discussed different approaches to repairing a RRD to achieve a high-integrity retinal attachment, highlighting the importance of reattaching the retina as closely as possible to its original location rather than reattaching the retina in a stretched state, which can result in what we refer to as a low-integrity retinal attachment.
Functional outcome measures
Functional outcome measures after RRD repair are essential because they provide a quantitative assessment for evaluating the success of different surgical techniques and provide information regarding prognosis. Past landmark studies have mainly used visual acuity as the primary measure of functional outcome, but in recent years investigators have had increased interest in the assessment of outcomes beyond VA, such as metamorphopsia and aniseikonia.
Many studies have incorporated objective assessment of these functional outcome measures after RRD repair, using M-CHARTS (Inami & Co.) to measure the degree of metamorphopsia5–7 and the Awaya new aniseikonia test (Handaya) for aniseikonia.8,9 Several authors have demonstrated that the incidence of metamorphopsia after RRD repair is significant, ranging from 56.7 to 88.6 percent.5,7,10,11 Similarly, aniseikonia has been reported in as many as 42 to 58 percent of patients post-RRD repair.8,9
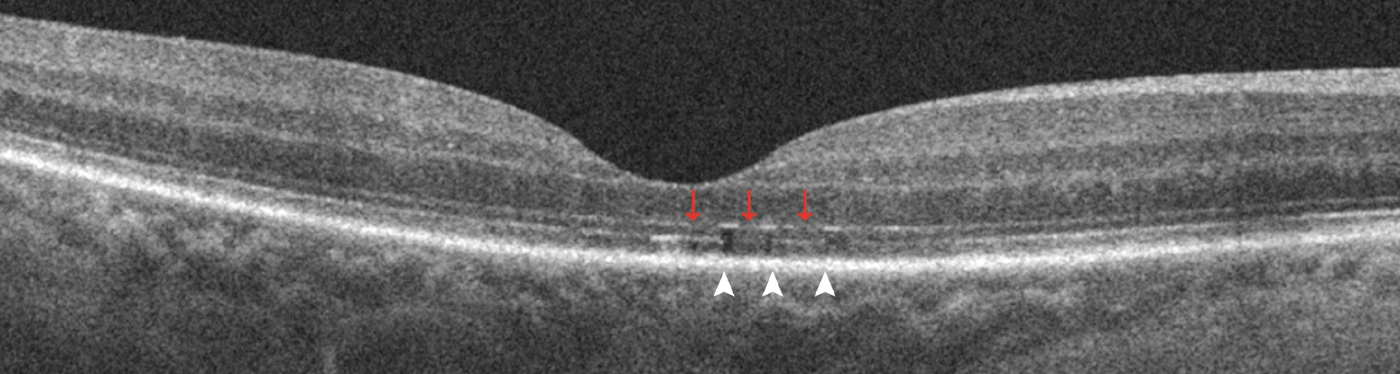 |
| Figure 1. Representative spectral-domain optical coherence tomography five-line raster images with discontinuity of the outer retinal bands three months after macula-off detachment repair. It shows discontinuity of the external limiting membrane (red arrows) and discontinuity of ellipsoid zone and interdigitation zone (white arrowheads) in the foveal scan. |
Role of multimodal imaging
Although metamorphopsia and aniseikonia are common following RRD repair, the pathophysiological basis is still not clearly understood due to conflicting evidence in the existing literature. Many contributing factors reduce functional outcomes after RRD repair, some of which are apparent on clinical examination. They include epiretinal membrane, cystoid macula edema or macula hole, but some can’t be explained by clinical examination alone.
Researchers at Tsukuba University in Japan reported that two-third of patients who had significant metamorphopsia exhibited normal macular contour with no identifiable gross abnormalities, such as ERM, CME, macular hole or persistent subretinal fluid, suggesting the existence of microstructural macular changes that standard fundus biomicroscopy couldn’t detect.7 In these cases, using multimodal imaging biomarkers has the potential to reveal important pathology that may explain the suboptimal functional outcomes.
Multimodal imaging has also given us a better understanding of the pathophysiology of RRD and its morphological stages,12 and the process of healing during reattachment.13 Imaging modalities are also used to visualize and comprehend the changes that the retina undergoes with different treatment modalities (i.e., PPV vs. pneumatic retinopexy [PnR],14 or laser retinopexy vs cryopexy15). Understanding these changes allows us to consider ways to improve or modify our surgical techniques to improve postoperative outcomes.
The following are the imaging modalities that can be useful in the management of RRD.
Optical coherence tomography
Several studies have used OCT to evaluate changes in the outer retina bands after RRD repair and have demonstrated the association of microstructural abnormalities with suboptimal functional outcomes.4,14,16,17 The microstructural imaging analysis of the fovea involved assessing for discontinuity of the outer retinal bands, specifically the external limiting membrane, ellipsoid zone and the interdigitation zone (IZ) (Figure 1, above), and the presence of outer retinal folds (ORFs) (Figure 2, below).
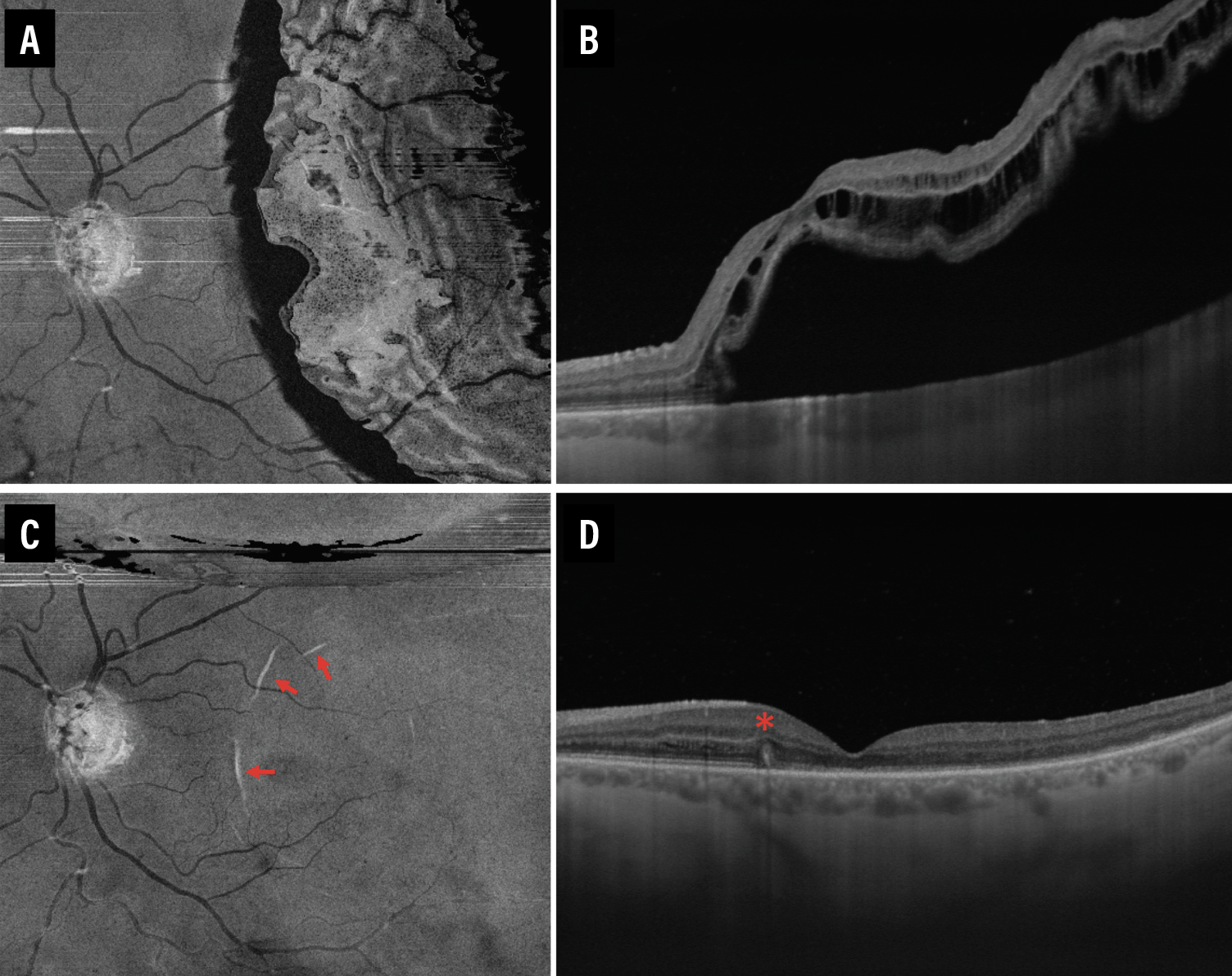 |
| Figure 2. En face and cross-sectional swept-source optical coherence tomography images of a patient before (A, B) and one week after (C, D) retinal detachment repair. Outer retinal folds are evidenced by the presence of hyper-reflective curvilinear lesions (red arrows) on the en face slab (C) with corresponding protrusions (red asterisk) on the cross-sectional (D) OCT scans of an eye that had recent RD repair. |
Researchers at Osaka University in Japan reported that postoperative integrity of the ELM and EZ are significant predictors of postoperative best-corrected visual acuity.4 Another team of Japanese researchers at Nagoya University supported these findings by demonstrating that BCVA correlated with thickness of the EZ to retinal pigment epithelium layer.17
Our group’s post-hoc analysis of a randomized controlled trial demonstrated that presence of ORFs was associated with worse visual acuity after RRD repair.16 Several other authors have demonstrated significant associations of metamorphopsia with outer retina abnormalities, such as ELM disruption5,11 and IZ discontinuity.5 Presence of aniseikonia were also associated with OCT changes. The Tsukuba University researchers in Japan demonstrated that a disrupted EZ was associated with aniseikonia.8
OCT angiography
OCT-A is a noninvasive way to both qualitatively and quantitatively assess the retinal vasculature following RRD repair. By enabling visualization of the superficial and deep capillary plexus (SCP and DCP), OCT-A also facilitates measurement of the foveal avascular zone (FAZ) size. Previous studies have shown that patients who undergo RRD repair exhibit FAZ enlargement at both the SCP and DCP compared to their contralateral normal eye.
Additionally, patients with macula-off RRD tend to have a larger FAZ than those with macula-on RRD.18 Increased FAZ represents ischemia, which correlates with worse visual acuity. This can be a useful biomarker to compare outcomes of different surgical techniques.
Ultra-widefield swept-source OCT
UWF-SS-OCT is a novel imaging system that delivers exceptional imaging capabilities for a wide range of retinal pathologies, extending even to the far periphery of the retina. In our recent study, we employed the newly available ultra-widefield confocal scanning laser ophthalmoscopy (CSLO) with single-capture integrated guided swept-source OCT to evaluate the retinal and choroidal response in the hours and days after laser retinopexy and cryopexy.15 We observed microstructural alterations consistent with chorioretinal adhesion almost immediately at one hour after laser retinopexy, whereas such changes were only apparent on postprocedure day six in the case of cryopexy.15
We can’t overstate the significance of these findings because they have a direct bearing on the success of RRD repair. The ability to achieve immediate adhesion following retinopexy can confer a distinct advantage, particularly in procedures such as PnR. UWF-SS-OCT also demonstrated that laser retinopexy was less traumatic, with preservation of the choroidal vasculature compared with cryopexy.
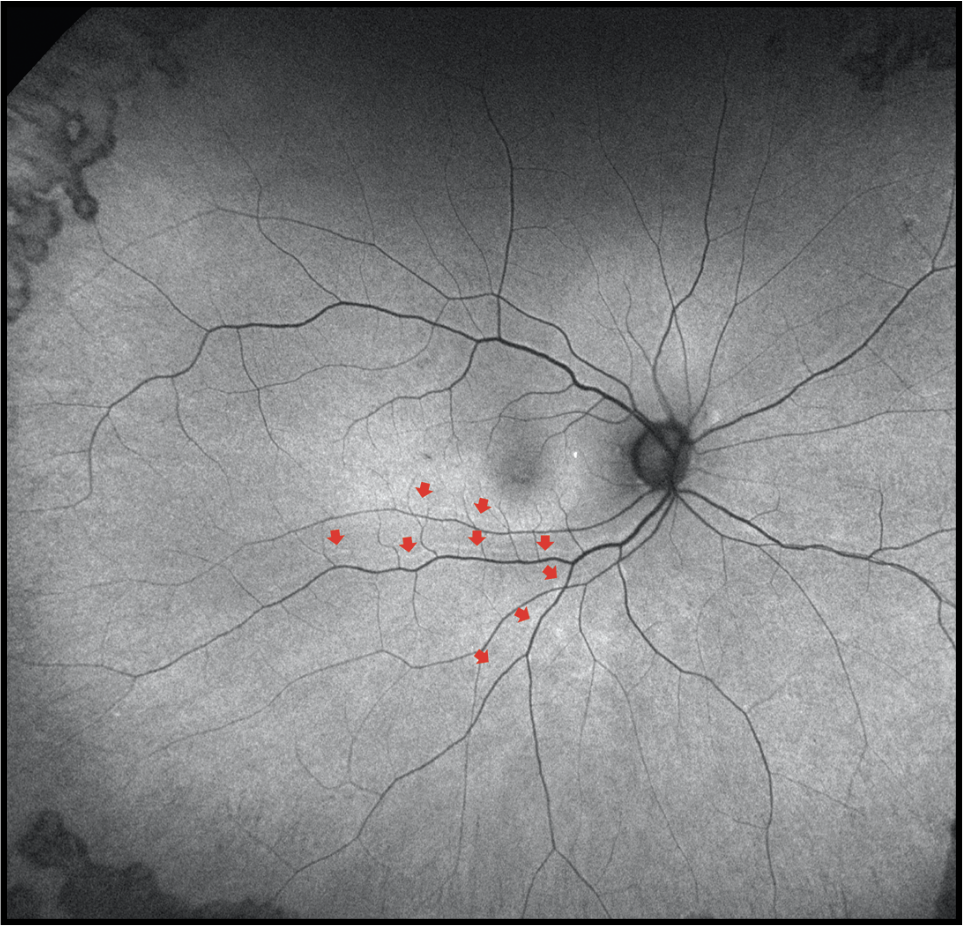 |
| Figure 3. Fundus autofluorescence three months after retinal detachment repair demonstrates anatomic reattachment with retinal displacement as evidenced by presence of retinal vessel printings (red arrows). |
Fundus autofluorescence
Researchers have shown recent interest in retinal displacement detected by the presence of retinal vessel printings on FAF imaging (Figure 3) after RRD repair. This is another important imaging biomarker that has been associated with suboptimal functional outcomes after RRD repair when OCT doesn’t find discernible abnormalities. Swiss investigators demonstrated that the presence of retinal displacement is associated with worse metamorphopsia.19 Another large study by our group illustrated that the presence of retinal displacement was associated with worse aniseikonia.20
Researchers in the United Kingdom found that the amplitude of retinal displacement was associated with worse BCVA.21 Because of these inconsistent findings, our group recently conducted a large comprehensive retrospective study that considered all imaging modalities and functional outcomes. Our findings indicated that the presence of retinal displacement had a significant impact on both BCVA and aniseikonia.22
Adaptive optics imaging
There’s limited information regarding the morphological and functional restoration of photoreceptors after undergoing retinal detachment repair. Adaptive optics (AO) imaging of the retina allows for en face visualization of individual cones and quantitative evaluation of the cone mosaic (Figure 4). AO imaging can be another important biomarker that allows assessment of individual cone photoreceptors that can’t be resolved with current commercial OCT technology due to its limited transverse resolution. This may be useful in assessing cone recovery and photoreceptor integrity after RRD repair.
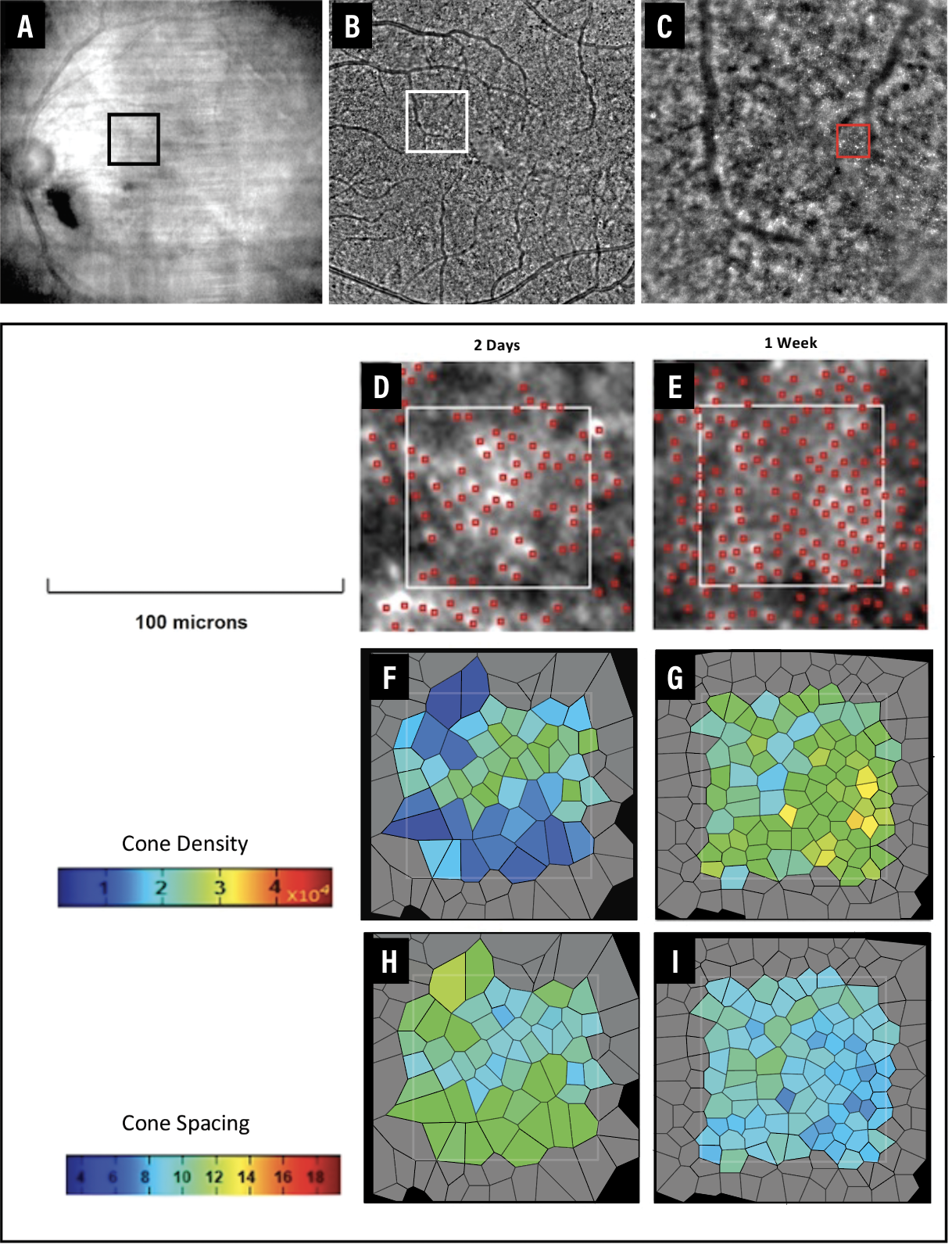 |
| Figure 4. Adaptive optics images of a patient with fovea-split retinal detachment demonstrate visible cones as early as one week after pneumatic retinopexy (PnR). A) Near-infrared image of the macula two hours after face- down positioning post-PnR. B) Montage shows visible cones one week after PnR. C) The cone mosaic at eccentricity 2 degrees superonasal to the fovea. The analyzed region of interest (D, E) demonstrates a progressive increase in cone density (F, G) and reduction in cone spacing (H, I) following PnR. (Colors may differ from those seen in the clinic due to printing limitations.) |
Several authors have described improvements in cone density after RRD repair with time but also noted that it was still significantly lower compared to the healthy fellow eye.23,24 Investigators have also demonstrated that cone density significantly correlated with visual acuity23 and mean retinal sensitivity measured with microperimetry,24 but no data to date exist on the association of changes to the cone mosaic with metamorphopsia or aniseikonia.
Increasing evidence suggests that the retina can be stretched, leading to retinal displacement following RRD repair, particularly related to the large gas bubble employed with PPV. Analysis of cone spacing with AO imaging may be a valuable imaging modality to provide additional information regarding changes to the photoreceptors immediately following RRD repair and its association with metamorphopsia or aniseikonia.
 |
Our group recently demonstrated that it’s possible to visualize and evaluate the macular cones using AO imaging as soon as one week after PnR.25 During the early stages of structural restoration following RRD repair, AO imaging may demonstrate cone mosaic recovery. Cone density increase and cone spacing reduction seen even before outer retinal band restoration is observed on OCT. AO imaging may make it possible to assess the photoreceptors more effectively during the initial phases of recovery following RRD repair. This could potentially facilitate comparative analyses of the cone mosaic across different surgical techniques.
Bottom line
In the current era of retinal detachment repair, we believe the definition of a successful repair has undergone a significant shift from simply achieving anatomic reattachment with the least number of procedures to now encompassing the attainment of structural integrity of the reattachment.
Multimodal imaging plays an essential role in achieving the integrity of the attachment because it allows for visualization of the physiological and pathophysiological reattachment, providing several novel biomarkers that can be used to assess outcomes and predict long-term prognosis for patients. These imaging biomarkers also provide useful outcome measures for future clinical trials that will compare various treatments for retinal detachment repair.
REFERENCES
1. Tornambe PE, Hilton GF. Pneumatic retinopexy. A multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment Study Group. Ophthalmology. 1989;96:772-783; discussion 784.
2. Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142-2154.
3. Hillier RJ, Felfeli T, Berger AR, et al. The Pneumatic Retinopexy versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial (PIVOT). Ophthalmology. 2019;126:531-539.
4. Wakabayashi T, Oshima Y, Fujimoto H, et al. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:519-528.
5. Okuda T, Higashide T, Sugiyama K. Metamorphopsia and outer retinal morphologic changes after successful vitrectomy surgery for macula-off rhegmatogenous retinal detachment. Retina. 2018;38:148-154.
6. Murakami T, Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Changes in metamorphopsia and optical coherence tomography findings after successful retinal detachment surgery. Retina. 2018;38:684-691.
7. Okamoto F, Sugiura Y, Okamoto Y, et al. Metamorphopsia and optical coherence tomography findings after rhegmatogenous retinal detachment surgery. Am J Ophthalmol. 2014;157:214–220.e1. .
8. Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Aniseikonia and foveal microstructure after retinal detachment surgery. Invest Ophthalmol Vis Sci. 2014;55:4880-4885.
9. Murakami T, Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Changes in aniseikonia and influencing-factors following successful macula-off retinal detachment surgery. Sci Rep. 2019;9:11588.
10. van de Put MAJ, Vehof J, Hooymans JMM, Los LI. Postoperative metamorphopsia in macula-off rhegmatogenous retinal detachment: Associations with visual function, vision related quality of life, and optical coherence tomography findings. PLoS One. 2015;10:e0120543–e0120543.
11. Zhou C, Lin Q, Chen F. Prevalence and predictors of metamorphopsia after successful rhegmatogenous retinal detachment surgery: A cross-sectional, comparative study. Br J Ophthalmol. 2016;101:725–729.
12. Martins Melo I, Bansal A, Naidu S, et al. Morphologic Stages of rhegmatogenous retinal detachment assessed using swept-source OCT. Ophthalmol Retina. 2022:S2468-6530.
13. Bansal A, Lee WW, Felfeli T, Muni RH. Real-time in vivo assessment of retinal reattachment in humans using swept-source optical coherence tomography. Am J Ophthalmol. 2021;227:265-274.
14. Muni RH, Felfeli T, Sadda SR, et al. Postoperative photoreceptor integrity following pneumatic retinopexy vs pars plana vitrectomy for retinal detachment repair: A post hoc optical coherence tomography analysis from the Pneumatic Retinopexy Versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial. JAMA Ophthalmol. 2021;139:620-627.
15. Lee WW, Muni RH. Single-capture ultra-widefield guided swept-source optical coherence tomography in the management of rhegmatogenous retinal detachment and associated peripheral vitreoretinal pathology. Br J Ophthalmol. 2022:bjophthalmol-2021-320149.
16. Lee WW, Bansal A, Sadda SR, et al. Outer retinal folds after pars plana vitrectomy vs. pneumatic retinopexy for retinal detachment repair: Post hoc analysis from PIVOT. Ophthalmol Retina. 2022;6:234-242.
17. Kobayashi M, Iwase T, Yamamoto K, et al. Association between photoreceptor regeneration and visual acuity following surgery for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci 2016;57:889–898.
18. Francisconi CLM, Kim DT, Juncal V, et al. Foveal avascular zone area analysis using OCT angiography after pneumatic retinopexy for macula-off rhegmatogenous retinal detachment repair. J Vit Ret Dis. 2019;3:297-303.
19. Schawkat M, Valmaggia C, Lang C, Scholl HP, Guber J. Multimodal imaging for detecting metamorphopsia after successful retinal detachment repair. Graefes Arch Clin Exp Ophthalmol. 2020;258:57-61.
20. Francisconi CLM, Marafon SB, Figueiredo NA, et al. Retinal displacement after pneumatic retinopexy versus vitrectomy for rhegmatogenous retinal detachment (ALIGN). Ophthalmology. 2022;129:458-461.
21. Casswell EJ, Yorston D, Lee E, et al. Effect of face-down positioning vs support-the-break positioning after macula-involving retinal detachment repair: The PostRD Randomized Clinical Trial. JAMA Ophthalmol. 2020;138:634-642.
22. Lee WW, Francisconi CLM, Marafon SB, Juncal VR, Hillier RJ, Muni RH. Multimodal imaging predictors of functional outcomes following rhegmatogenous retinal detachment repair. Paper presented at the Association of Research in Vision and Ophthalmology annual meeting 2022; Denver, CO; May 1–4, 2022.
23. Potic J, Bergin C, Giacuzzo C, et al. Changes in visual acuity and photoreceptor density using adaptive optics after retinal detachment repair. Retina. 2020;40:376-386.
24. Reumueller A, Wassermann L, Salas M, et al. Morphologic and functional assessment of photoreceptors after macula-off retinal detachment with adaptive-optics OCT and microperimetry. Am J Ophthalmol. 2020;214:72-85.
25. Lee WW, Oquendo P, Muni RH. Early adaptive optics imaging after rhegmatogenous retinal detachment repair. Am J Ophthalmol. 2023;247:e3.



