The milieu of MIGS comprises several different procedures and devices, targeting
 |
The Conundrum of Glaucoma
In upcoming decades, the prevalence of glaucoma is only expected to increase as the population ages.1 Of the various forms of glaucoma—acute and chronic angle closure, secondary open-angle, secondary angle closure, juvenile and congenital—primary open-angle glaucoma (POAG) is by far the most common, accounting for approximately 74 percent of all cases worldwide.2
The Ocular Hypertension Treatment Study (OHTS) outlined importance of early intraocular pressure (IOP) control in the treatment of glaucoma.3 OHTS showed that eyes with untreated ocular hypertension have a 9.5 percent rate of glaucomatous progression over five years, and decreasing IOP by 20 percent reduces the rate of progression to 4.4 percent. Treatment for mild/moderate POAG has traditionally focused on eye drops and selective laser trabeculoplasty (SLT). The safety profile of these treatments is favorable, but the real-world efficacy less so.
Noncompliance with glaucoma regimens is notoriously widespread, with rates ranging from 25 to 80 percent depending on the number of medications and drop frequencies.4 The problem is multifactorial, as ocular side effects and rising medication costs also contribute to poor compliance. While
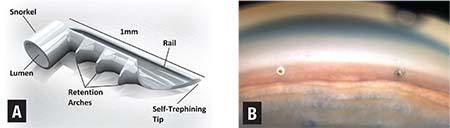 |
| Figure 1. A schematic of iStent (Glaukos) (A) and a gonioscopic image (B) of the iStent successfully implanted in the nasal trabecular meshwork. |
Aqueous Outflow Pathways
The ciliary body epithelium produces aqueous humor in the posterior chamber and flows through the pupil into the anterior chamber. The aqueous exits the eye either through the trabecular meshwork (TM) into Schlemm’s canal and aqueous veins (conventional pathway) or through the ciliary muscle and other downstream tissues (uveoscleral pathway).
In the conventional
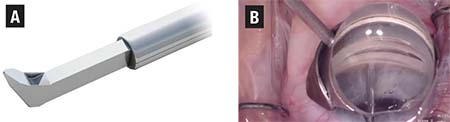 |
| Figure 2. Schematic (A) shows the proprietary footplate of the Kahook Dual Blade (KDB) designed to simultaneously raise and excise trabecular meshwork tissue, and a gonioscopic image (B) of the KDB advancing across the nasal trabecular meshwork. |
In the uveoscleral outflow pathway, aqueous humor enters the ciliary muscle and exits through the supraciliary space and across the anterior or posterior sclera, through the emissarial canals around the vortex veins or into the choroidal vessels. We now know that uveoscleral drainage can account for up to 60 percent of the total aqueous humor drainage.8 MIGS devices that focus on the uveoscleral pathway include the CyPass (Alcon, Figure 3) and, to an extent, the XEN45 gel stent (Allergan).
Targeting Conventional Pathway
• iStent. Approved by the Food and Drug Administration in 2012, the Glaukos iStent (Figure 1A) was one of the first MIGS devices to reach the U.S. market. It is an L-shaped trabecular stent made of heparin-coated titanium, and it is the smallest MIGS device at 0.3 mm by 1 mm. The device is inserted via an ab interno approach through the trabecular meshwork and seated into Schlemm’s canal in order to increase aqueous outflow. Currently in the United States, iStent is only approved for implantation at the time of cataract surgery.
Initial efficacy studies are promising. In 2015, a study comparing cataract surgery combined with iStent implantation to cataract surgery alone showed that 68 percent of patients in the iStent treatment group met the primary endpoint of IOP < 21 mmHg with no medications at one year, compared with only 50 percent of subjects receiving cataract surgery only (p=0.004).9 A subsequent meta-analysis including 2,495 patients in more than 30 studies concluded that combined cataract surgery and iStent implantation reduced IOP by 9 percent compared with a 4 percent reduction after cataract surgery alone.10
A newer-generation iStent available in Europe and other countries has been modified to allow for the implantation of two stents in an eye during the same procedure. A meta-analysis of eyes receiving two
iStents showed a 27-percent reduction in IOP from preoperative baseline.10 Safety profiles for combined cataract surgery/iStent implantation are similar to cataract surgery alone, and given that aqueous outflow through the trabecular pathway is limited by episcleral venous pressure, hypotony is extremely rare.10
Postoperative care for patients receiving iStent implantation does not differ significantly from standard cataract surgery in terms of drop schedules and follow-up, although a final steady-state reduction in IOP may not occur for several weeks.
• Kahook Dual Blade (KDB). Introduced in the United States in late 2015, the KDB (Figure 2) is a single-use
 |
| Figure 3. Depiction of the CyPass Micro-Stent (A) shunting aqueous fluid into the suprachoroidal space (A), and a gonioscopic image (B) of the CyPass post-implant. |
A multicenter cohort study of 122 eyes at eight centers revealed that combined cataract surgery with the KDB procedure yielded an average postoperative IOP of 13 mmHg compared to the preoperative baseline of 17.4 mmHg (p<0.001).11 The mean glaucoma medication burden was also significantly reduced compared to baseline (p<0.001). In 96 percent of cases, surgeons agreed or strongly agreed that the use of the KDB was straightforward, entry into Schlemm’s canal was uncomplicated, and advancement along the treatment pathway was without difficulty.
As with the iStent, postoperative care for the KDB is similar to standard cataract surgery.
Targeting Uveoscleral Outflow
• CyPass Micro-Stent. One of the newer MIGS devices, having been approved in summer 2016, the CyPass Micro-Stent (Figure 3) is a fenestrated microstent 6.35 mm long and 500 μm in diameter, composed of biocompatible polyimide material that is magnetic resonance safe. It is inserted into the angle between the scleral spur and ciliary body, creating a direct communication between the anterior chamber and the suprachoroidal space. It is indicated for placement at the time of cataract surgery to treat mild-to-moderate OAG.
The multicenter, randomized COMPASS trial enrolled more than 500 patients.12 Preoperatively, all patients had mild-to-moderate POAG with unmedicated IOP between 21 and 33 mmHg. At the two-year endpoint, patients receiving phacoemulsification combined with CyPass implantation showed an average drop in IOP of 7 mmHg compared with 5.3 mmHg in the control group receiving phacoemulsification alone. In addition, 93 percent of patients in the CyPass group remained medication-free at two years. No severe adverse events occurred throughout the study compared with cataract surgery alone. While hypotony is a theoretical concern in any device that shunts to the suprachoroidal space, the COMPASS investigators did not report any clinical hypotony. As with other MIGS procedures, postoperative care is no different than with standard cataract surgery.
| Take-home Point The advent of minimally invasive glaucoma surgery—MIGS—provides an alternative to drop therapy with a higher level of efficacy while making minimal compromises in overall safety. This article explores the pathophysiology of glaucoma, and reviews the MIGS devices available in the United States and their mechanisms of action. Moving forward, the ever-expanding range of devices and procedures affords the opportunity to further customize glaucoma treatment to the individual and at the same time greatly reduce the burden of patient compliance. |
Targeting Other Mechanisms
• Endoscopic Cyclophotocoagulation (ECP). ECP attempts to decrease the rate of aqueous production in the ciliary body epithelium by using a curved endoscopic probe that contains a light source, a camera and a semiconductor diode laser. This technology allows direct visualization of the ciliary epithelium, allowing the laser energy to be precisely delivered to the ciliary processes for 270° to 360°, thus limiting damage to the underlying ciliary body and surrounding tissues. For this reason, it is widely accepted as much safer than other cyclodestructive procedures. In the United States, it is performed in conjunction with cataract surgery.
A randomized controlled trial in 626 medically controlled glaucoma patients reported that phacoemulsification combined with ECP was superior to phacoemulsification alone in lowering IOP and decreasing glaucoma medication burden.13 These authors reported no serious complications in either group, and the rates of cystoid macular edema were the same in both groups. Postoperative care does not differ significantly from that of standard cataract surgery.
• Xen Gel Stent. This is another one of the newer MIGS devices in the United States, having been approved in the fall of 2016. Xen Gel is a soft, permanent, subconjunctival implant approximately 6 mm long derived from a collagen-based, noninflammatory gelatin. It is inserted using a disposable Xen injector through a small, self-sealing corneal incision into the subconjunctival space via an ab interno approach. Flexible material allows it to conform to ocular tissue, possibly minimizing many of the issues seen with synthetic materials such as migration, erosion and corneal endothelial damage. While the Xen Gel Stent has the potential to benefit a wide range of patients, it is currently marketed for the treatment of refractory glaucoma.
A trial of refractory glaucoma patients showed that the Xen Gel reduced IOP from a mean medicated baseline of 25.1 (+ 3.7) mmHg to 15.9 (+ 5.2) mmHg at 12 months postoperatively.14 The mean baseline number of IOP-lowering medications was 3.5 vs. an average use of 1.7 at 12 months. Following surgical implantation, providers will need to be comfortable with issues regarding bleb management in addition to standard postoperative cataract care. RS
REFERENCES
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393.
2. Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;1224:532–538.
3. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701-713.
4. Olthoff CM, Schouten JS, Van De Borne BW, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: An evidence-based review. Ophthalmology. 2005;112:953-961.
5. De Keyser M, De Belder M, De Belder S, De Groot V. Where does selective laser trabeculoplasty stand now? A review. Eye Vis (London). 2016;3:10.
6. Gross RL. Glaucoma filtration surgery, trabeculectomy or tube shunt? Am J Ophthalmol. 2012;153:787-788.
7. Grant WM. Further studies on facility of flow through the trabecular meshwork. Arch Ophthalmol. 1958;60:523–533.
8. Alm A, Nilsson SF. Uveoscleral outflow—a review. Exp Eye Res. 2009;88:760–768.
9. Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term results. J Cataract Refract Surg. 2015;41:2664-2671.
10. Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: A meta-analysis. PLoS ONE. 2015;10(7):e0131770.
11. Abdullah S, Jasek MC, Radcliffe NM, et al. A novel dual blade device for goniotomy: initial clinical experience. Invest Ophthalmol Vis Sci. 2016;57:6522.
12. Vold S, Ahmed IIK, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103-2112.
13. Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40:1313–1321.
14. Dapena CL, Ros RC. Glaucoma-filtering surgery with a XEN® collagen-based implant via the ab interno route [in Spanish]. Revista Española de Glaucoma e Hipertensión Ocular. 2015;5:350-357.



