 |
 |
 |
 |
As retina specialists, we readily agree that the introduction of anti-VEGF agents for a variety of retinal diseases has helped us preserve sight for countless patients. However, for treatment of chronic conditions, such as neovascular age-related macular degeneration that affects our elderly population, we also know more work is needed to reduce the treatment burden while maintaining visual acuity.
In nAMD, approximately 41 percent of newly diagnosed and treated patients will gain at least 3 lines of vision, but others may never achieve 20/40 or better VA even with consistent treatment.1-3 Mounting evidence may help our understanding of this conundrum. Studies show that baseline VA at the time of diagnosis is strongly associated with vision outcomes with intravitreal anti-VEGF agents.4-6 Therefore, diagnosing and treating patients earlier in the disease process could be a key to improving visual outcomes in nAMD.
We know from numerous clinical trials and published reports on nAMD that frequent and consistent intravitreal injections with anti-VEGF agents and close monitoring for progression or relapse are critical in maintaining VA and vision gains. Ultimately, these require continued in-office visits.
While office visits were already challenging for some elderly patients under the best circumstances—transportation, for example, can be difficult for them to arrange—they’ve become substantially more so in the wake of the COVID-19 pandemic as the elderly population is at an increased risk for complications from the virus. As we slowly reopen our offices and practice under the “new normal” methods prioritizing the safety of our patients and staff, retina specialists need to consider alternative ways of providing high-quality care. One such way is through remote monitoring and telehealth.
Home-based OCT
 |
Optical coherence tomography improves the diagnosis and management of many retinal conditions. This is especially true with nAMD, for which diagnosis and treatment decisions, including treatment interval, depend heavily on OCT findings in the clinic.
What if we could perform OCT in the patient’s home? A new OCT system that patients can use at home rather than in the office is in development (Figure 1, page 25, Home OCT, Notal Vision). This patient-operated OCT system is designed to support and enhance standard of care. Patients with nAMD use the device at home to capture macular images, which are then relayed to the treating physician and the remote diagnostic center to identify fluid recurrence or progression and to provide inter-visit disease information.
The diagnostic clinic provides the device to the patient following a physician referral. The remote clinic educates the patient about device use, monitors patient self-imaging compliance and can send reminders if the patient isn’t self-monitoring. Thus, home-based OCT may be a solution for reducing the burden of coming to the office for patients while they’re being safely monitored remotely. It may also increase the chances of timely detection of recurrent fluid and prompt a visit for intravitreal injection, thus reducing the risk of VA loss.
Our experience evaluating the device
My colleagues and I recently evaluated the investigational Home OCT in patients with either nAMD or non-nAMD to assess its ease of use and the overall experience of operating the system for self-imaging by the patients. We also compared the device to in-office OCT imaging for disease activity.
We conducted a company-sponsored prospective study of 531 consecutive eyes (290 patients) with nAMD and non-nAMD with VA better than or equal to 20/400. The average patient age was 79 years with a mean VA of 20/40; 17 percent had VA of 20/80 or worse. Photographers imaged patients without dilation using a commercial OCT (388 eyes were imaged with the Cirrus [Zeiss] and 81 eyes with the Spectralis [Heidelberg Engineering]). The patients then viewed a two-minute video tutorial of the Home OCT, followed by self-operating the device to capture images of their own eyes. Those images were then compared to the commercial OCT images by a masked reader to determine the presence of fluid.
The results were encouraging, with 91 percent of patients able to complete the Home OCT imaging in at least one eye. We found that VA had a role in successful imaging: 81 to 93 percent of patients with VA >20/320 were able to capture successful images compared to only 50 percent of those with <20/320. More than 90 percent of patients agreed or strongly agreed that the video tutorial was helpful and clear and that the scan was quick, comfortable and easy. A total of 46 percent of eyes had fluid. Thirteen eyes had a discrepancy in image reading between the Home OCT and commercial OCT; positive and negative agreement were 98 and 96 percent, respectively.
I should note, however, that although the Home OCT was designed for home use, the study was done in an in-office setting, so we don’t yet have real-world experience with the device. Based on our in-office experience, though, and coupled with the use of a remote diagnostics clinic that will follow-up with patients, I don’t expect home use to be much different from what we saw in our study.
As the next step in this research, the first longitudinal at-home study started in June. It demonstrates for the first time, the feasibility of patient daily OCT self-imaging, cellular transmission of data to the cloud for image analysis and remote physician review. We eagerly await the study results.
 |
AI supports treating physicians
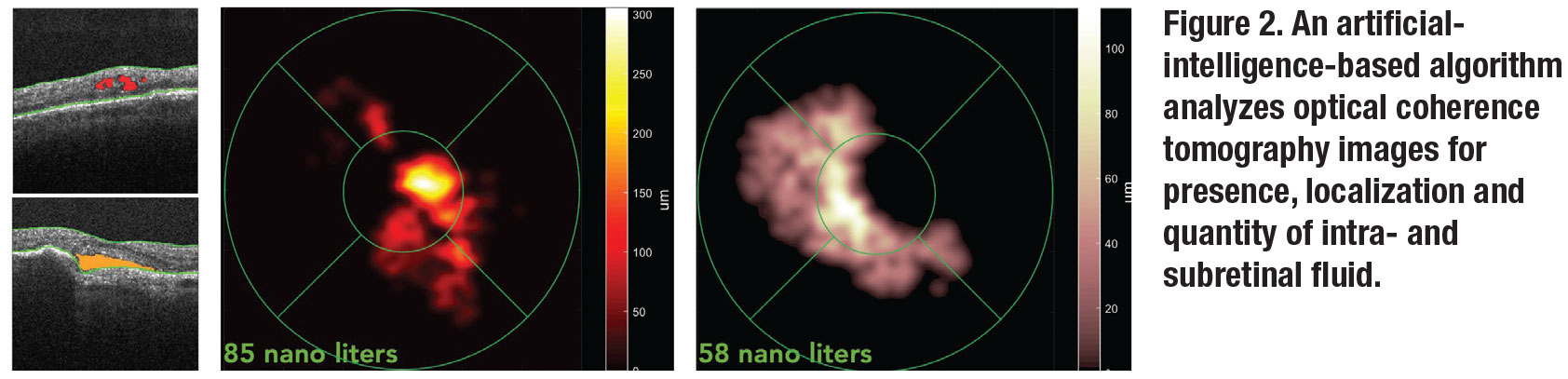
To cope with the large amount of daily Home OCT images that require analysis, an artificial-intelligence-based algorithm that identifies, localizes, quantifies and maps fluid on each scan has been developed (Figure 2). It can track intra- and subretinal fluid over time and enables the physician to set thresholds for sending automated alerts. We can look at fluid in isolation regardless of retinal alterations like fibrosis that can mask disease activity on retinal thickness maps.
Real-world impact on patients
Despite the study limitations, my colleagues and I believe the Home OCT can provide valuable information to patients and physicians on disease progression, helping to support retina specialists to better personalize AMD management, maintain VA throughout the treatment course and reserve in-clinic visits for injections rather than routine monitoring.
I also believe that this and similar devices may improve our follow-up rates. Some patients with AMD may end up with fewer visits, resulting in fewer transportation challenges and hopefully less treatment burnout. Others will be more motivated to come in knowing that they have fluid that needs to be treated.7,8 Being able to better identify patients needing more frequent injections compared to those needing fewer injections will benefit us all.
 |
An aid for the ‘new normal’
We’re still trying to navigate “normal” office procedures in the wake of COVID-19. This may include limiting in-office imaging. However, devices that give us the flexibility to monitor patients remotely allow us to stay connected with them and ensure we are still providing the highest quality care when they need to be in our offices.
Remote diagnostic clinics providing monitoring services, including compliance of home use of the device, are important partners in patient comanagement in this new era. Also, in this model we can hand off time-consuming patient education tasks of how to utilize the system to the phone service of a remote provider, reducing the time a patient spends in our clinics.
At the same time, the ability to remotely review images by retina specialists could allow us to engage directly with our patients on telehealth consultations. Extreme times such as these require innovative thinking and adaptability to provide the best care. Technologies such as Home OCT may help us to do so as we move forward. If all goes well, the remote monitoring service is expected to be available in the United States by prescription in the first half of 2021. RS
REFERENCES
1. Rosenfeld PJ, Brown DM, Heier JS, et al., for the MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.
2. Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014;121:193-201.
3. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-1444.
4. Ying GS, Huang J, Maguire MG, et al, for the Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology 2013;120:122-129.
5. Maguire MG, Martin DF, Ying GS, et al, for the Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:1751-1761.
6. Ying GS, Maguire MG, Daniel E, et al, for the Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Association of baseline characteristics and early vision response with 2-year vision outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2015;122:2523-2531 e1.
7. Obeid A, Gao X, Ali FS, et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol 2018;136:1251-1259.
8. Prenner JL, Halperin LS, Rycroft C, et al. Disease burden in the treatment of age-related macular degeneration: Findings from a time-and-motion study. Am J Ophthalmol 2015;160:725-731.e1.



