 |
Initially dubbed the vision impairment/intracranial pressure (VIIP) syndrome, the symptoms and signs were thought to be related to increased intracranial pressure resulting from spaceflight-associated factors: fluid shifts; elevated ambient carbon dioxide concentrations; resistive exercise and/or dietary sodium. Elevated intracranial pressure could impinge on the optic nerve and eye.
Since reports of the original seven cases of VIIP in 2011, much has been done and even more has been written in an attempt to characterize this syndrome, find a ground-based analog in which to study it, and/or propose preventative measures and treatments.1-9 However, not all astronauts are affected, and the number of cases has varied from mission to mission and year to year, leading to a debate over the actual incidence of ocular issues in ISS astronauts.
A recent consensus has coalesced around the idea that all ISS inhabitants have some degree of optic disc edema during and/or after flight, and that this edema reaches clinical significance in about 15 to 20 percent of them. We reported that 13 percent of astronauts had cotton-wool spots, 60 percent had a change in visual acuity of at least 0.5 D in one eye, 13 percent had choroidal folds and 65 percent had globe flattening.10 Nonetheless, many astronauts do not develop any of these findings.
One-Carbon Metabolism
As part of a study examining nutritional status, we were analyzing a broad range of nutritional and biochemical analytes from astronauts before, during and after flight. We had analytes for five of the seven VIIP cases, and found that astronauts who had ophthalmic changes also had higher concentrations of homocysteine than those who did not. At that point when we were analyzing our data, we classified VIIP cases dichotomously; either they had any of the previously mentioned ophthalmic changes after flight or they had none. Affected astronauts also had higher concentrations of three other metabolites: cystathionine; 2-methylcitric acid; and methylmalonic acid.
What’s more, these four metabolites were at higher concentrations in affected astronauts before flight. These astronauts also had significantly lower serum folate concentrations during flight. 11
We hypothesized that genetics might be responsible for the differences in circulating metabolites of one-carbon metabolism between affected and unaffected astronauts. One-carbon metabolism is replete with common gene variants that influence the activity of enzymes in the pathway. Some of these gene variants are associated with subtle elevations in homocysteine and other metabolites and reductions in serum folate, all of which we had observed in affected astronauts.
In 49 astronauts who participated in a small study of five single-nucleotide polymorphisms (SNPs), one being the gene for the enzyme methionine synthase reductase (MTRR, rs1801394) A66G SNP, all who had the GG gene variant (rather than the AG or AA variant) of this SNP had ophthalmic findings (Figure 1). However, not everyone with ophthalmic issues had this variant of the gene. Statistical modeling of the data demonstrated that specific
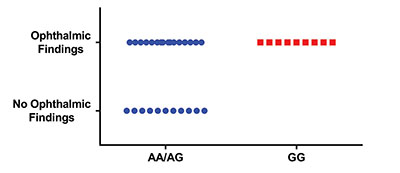 |
| Figure 1. Ophthalmic results (number of astronauts having ophthalmic findings or not) in astronauts having different single-nucleotide polymorphisms (SNPs) in their gene for the enzyme methionine synthase reductase. Astronauts having the AA or AG variants of the SNP were combined for comparison with astronauts having the GG variant. |
Our extended biochemical, endocrine and metabolomic analyses also identified associations of ophthalmic issues with androgens and carbohydrate (glucose-mannose) metabolism markers.10 For example, individuals with a greater testosterone response (area under the curve for testosterone concentration) during spaceflight had a greater dioptric change. Those with higher preflight concentrations of dehydroepiandrosterone-sulfate (DHEA-S, a testosterone precursor) had a higher prevalence of cotton-wool spots and a greater dioptric change after flight.
Thus, we have established an association of one-carbon pathway genetics and B-vitamin status with the development of ophthalmic pathology in some astronauts.10 However, we cannot explain the etiology of every case of ophthalmic pathology, in part because we evaluated such a small number of SNPs; only five. To remedy that, we have initiated an experiment to evaluate a much broader set (>500) of SNPs associated with one-carbon metabolism in astronauts.
The Hypothesis
We developed an end-to-end hypothesis to describe how one-carbon pathway genetics and B-vitamin status might be related to ophthalmic changes in some astronauts.12 We proposed a multiple-hit hypothesis linking endothelial dysfunction, leaky blood vessels and subclinical edema. Several insults lead to vascular endothelial dysfunction by way of impaired nitric oxide synthesis and oxidative stress. Genetic along with biochemical, environmental and physiological factors in some combination cause impaired endothelial nitric oxide synthesis and oxidative stress. These lead to endothelial dysfunction, leaky blood vessels and subsequent subclinical edema. Localized edema in the cerebral venous sinuses impinges upon arachnoid villi, reducing the normal efflux of cerebrospinal fluid (CSF) from the villi into the venous circulation. Pressure from the slowed CSF efflux to the venous sinuses builds up in the subarachnoid space of the optic nerve and exerts itself on the optic nerve and the posterior globe.12 Individual structural variations of the area surrounding the optic canal could exacerbate this increased pressure.12 The subarachnoid space of the optic nerve is narrowest around the optic canal.
Genetics can affect individual nutrient requirements, and subsequently peripheral and cerebral vitamin status. The function of the blood-brain barrier (BBB) can further affect this. The BBB concentrates certain vitamins, including folate, in the brain using active transport mechanisms. Anything that affects BBB permeability can thus affect cerebral vitamin status. Radiation, a key concern for space travelers, can also affect BBB function. Radiation and B-vitamin deficiencies are also associated with an increased incidence of factors such as white matter hyperintensities, which are currently being studied in astronauts.
B-vitamin insufficiency is associated with increased serum homocysteine and depleted reserves of endothelial antioxidants. Oxidative stress, in combination with other physiological (e.g., fluid shifts, altered testosterone or carbohydrate metabolism) or environmental (e.g., radiation, ambient CO2) influences, contributes to endothelial dysfunction.
Fluid shifts, radiation, CO2 and oxidative stress may lead to endothelial dysfunction and contribute to astronauts’ ophthalmic pathology. Yet, not all astronauts develop ophthalmic pathology. Thus, we maintain that genetic effects on one-carbon pathway function remain an indispensable factor in this pathology, given that all astronauts are exposed to microgravity-induced fluid shifts, radiation and cabin CO2 concentrations higher than those on Earth.
However, testing this hypothesis is challenging given the inherent difficulties of obtaining these data during spaceflight and the lack of ground-based environments that simulate spaceflight. Some have reported small ophthalmic changes during bed rest, a common analog for effects of spaceflight on bone and muscle, but nothing to the extent observed in astronauts.4,13
Astronauts and Ovaries
One of the more intriguing observations related to the phenomenon of ocular changes in spaceflight is that many documented or purported characteristics of astronauts with ophthalmic changes are shared by women with polycystic ovary syndrome (PCOS) (Figure 2). Curiously, all of the frank cases of spaceflight-related ophthalmic pathology to date have been in men, and debate surrounds whether the condition affects only men or if we simply haven’t flown enough women. While having ovaries is a requirement for being diagnosed with PCOS,
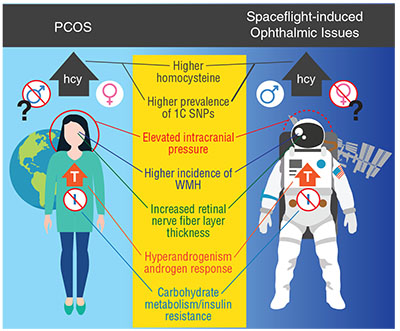 |
| Figure 2. This graphic compares findings in polycystic ovary syndrome (PCOS) and spaceflight-induced ophthalmic issues. Only women are thought to have PCOS, but so far only men have had spaceflight-induced ophthalmic issues. Commonalities in the two conditions include elevated serum homocysteine concentrations, greater prevalence of risk alleles in the one-carbon metabolic pathway, higher incidence of white-matter hyperintensity, increased retinal nerve fiber layer thickness, elevated testosterone levels (in PCOS) or response of testosterone to spaceflight, altered carbohydrate metabolism, insulin resistance and possibly elevated intracranial pressure. |
We are currently evaluating one-carbon genetics/biochemistry and ophthalmic exams in women with PCOS and/or idiopathic intracranial hypertension (IIH), patients with IIH (but not PCOS) and matched controls. Our hypothesis is that the similarities between women with PCOS and astronauts with ophthalmic issues are real, and that women with PCOS may provide a ground analog population to study these genetic/ocular relationships and hopefully lead to countermeasures.
Ultimately, proving this hypothesis could yield substantial benefits for both aerospace and terrestrial medicine. Specifically, the study will shed light on the understanding of the nature and genetics of PCOS. Understanding these processes more thoroughly provides the opportunity to find new treatments, or make current treatments more effective.
For one intriguing anecdote, when we published our genetics paper in 2016, a fellow NASA employee emailed, stating, “Six years ago I was diagnosed with papilledema that has caused my right pupil to dilate differently from my left. No cause was ever found. Something is putting pressure on my optic nerve … but what? At some point in all of this, I was diagnosed with a B12 deficiency. And I am one of those 10 to 20 percent of women with PCOS.”
The Countermeasure
Preventing or treating ophthalmic issues during spaceflight is critical before we embark on exploration-class missions far from Earth. The most obvious and lowest-risk countermeasure, according to our data and subsequent hypothesis, is a biologically active B-vitamin supplement.
Pharmacologic agents plausibly might have their specific effects on points in this hypothesis, but might also miss others with unknown consequences. B-vitamin supplementation has been shown to enhance nitric oxide synthesis, mitigate endothelial dysfunction, improve blood-brain barrier function and reduce hypertension, among other things—all with very low risk.
But we have yet to prove our hypothesis, so perhaps it is imprudent to prescribe vitamins at this point. We feel that evidence must be established. Nonetheless, we have documented an association between one-carbon genetics, B-vitamin status and astronauts who develop ophthalmic issues. We recently also documented that individuals with one-carbon genetics similar to that of astronauts who develop ophthalmic issues have altered responses to CO2 exposure,16 so this line of thinking may not be a fluke. One could argue that our hypothesis is wrong, and that an entirely different set of arrows could link one-carbon genetics and B-vitamin status to these ophthalmic issues. That is the point of research.
Biomedical research is a slow process, but we expect that developing our hypothesis, whether it proves true or not, has produced ideas that will contribute to solving problems in several areas of medicine.
Take-home Point
The genetics of one-carbon metabolism and B-vitamin status have been associated with the development of ophthalmic changes in astronauts after missions on the International Space Station. This article describes a hypothesis that explains how genetics and physiological and biochemical insults to vascular endothelial function result in impaired nitric oxide synthesis and oxidative stress, and lead to endothelial dysfunction and a buildup of pressure around the optic nerve and the posterior globe. Furthermore, individual structural variations of the area surrounding the optic canal could exacerbate the effects of increased pressure. An additional hypothesis holds that the genetics, biochemistry and cardiovascular (and potentially ocular) physiology are similar between affected astronauts and women with polycystic ovary syndrome. The implications of this research for both space and terrestrial medicine are profound.
Acknowledgments: The authors would like to acknowledge the One- Carbon Investigator Team: Drs. Jesse F. Gregory, Steven H. Zeisel and Patrick J. Stover for their work in B vitamins and one-carbon metabolism; Drs. Thomas Mader and C. Robert Gibson for their experience in evaluating astronaut eyes; and Drs. Alice Y. Chang and John J. Chen for leading the efforts to accomplish our study of one-carbon genetics, biochemistry and ocular exams in women with polycystic ovary syndrome. Acknowledgment also goes to the astronauts who have participated from the outset and Jane Krauhs for editorial assistance. The Human Health Countermeasures Element of NASA’s Human Research Program and the Nutritional Biochemistry Laboratory Team have also supported the research.
REFERENCES
1. Lee AG, Tarver WJ, Mader TH, Gibson CR, Hart SF, Otto CA. Neuro-ophthalmology of space flight. J. Neuroophthalmol. 2016;36:85-91.
2. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema in an astronaut after repeat long-duration space flight. J. Neuroophthalmol. 2013;33:249-255.
3. Marshall-Bowman K, Barratt MR, Gibson CR. Ophthalmic changes and increased intracranial pressure associated with long duration spaceflight: an emerging understanding. Acta Astronaut. 2013;87:77-87.
4. Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G. The effect of microgravity on ocular structures and visual function: a review. Surv. Ophthalmol. 2013;58:155-163.
5. Marshall-Goebel K, Mulder E, Bershad E, et al. Intracranial and intraocular pressure during various degrees of head-down tilt. Aerosp Med Hum Perform. 2017;88:10-16.
6. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058-2069.
7. Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595:2115-2127.
8. Mader TH, Gibson CR, Otto CA, et al. Persistent asymmetric optic disc swelling after long-duration space flight: Implications for pathogenesis. J. Neuroophthalmol. 2017;37:133-139.
9. Mader TH, Gibson CR, Lee AG, Patel NB, Hart SF, Pettit DR. Unilateral loss of spontaneous venous pulsations in an astronaut. J. Neuroophthalmol. 2015;35:226-227.
10. Zwart SR, Gregory JF, Zeisel SH, et al. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes. FASEB J. 2016;30:141-148.
11. Zwart SR, Gibson CR, Mader TH, et al. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J. Nutr. 2012;142:427-431.
12. Zwart SR, Gibson CR, Gregory JF, et al. Astronaut ophthalmic syndrome. FASEB J. 2017;31:3746-3756.
13. Taibbi G, Cromwell RL, Zanello SB, et al. Ocular outcomes comparison between 14- and 70-day head-down-tilt bed rest. Invest Ophthalmol Vis. Sci. 2016;57:495-501.
14. Kurzrock R, Cohen PR. Polycystic ovary syndrome in men: Stein-Leventhal syndrome revisited. Med Hypotheses. 2007;68:480-483.
15. Yanes Cardozo LL, Romero DG, Reckelhoff JF. Cardiometabolic features of polycystic ovary syndrome: role of androgens. Physiology. 2017;32:357-366.
16. Laurie SS, Vizzeri G, Taibbi G, et al. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep. 2017;5:e13302.



