| New Insights into Gene Therapy |
Take-home points
|
 |
|
Bios Mr. Qin is a medical student at the Medical College of Georgia, Augusta University, Augusta. Dr. Kasetty is a second-year ophthalmology resident at Henry Ford Hospital in Detroit. Dr. Marcus is a vitreoretinal surgeon and managing physician at Southeast Retina Center, director of clinical research at Eye Health America, and professor of clinical ophthalmology, Medical College of Georgia, Augusta University. Dr. Espinosa-Heidmann is a vitreoretinal surgeon, associate professor and retina fellowship director at the department of ophthalmology at the Medical College of Georgia, Augusta University. DISCLOSURES: Mr. Qin, Dr. Kasetty and Dr. Espinosa-Heidmann have no disclosures. Dr. Marcus disclosed serving as a consultant to RegenxBio, Genentech/Roche, Regeneron Pharmaceuticals and Vial, and receiving research grants from Allergan/AbbVie, Aiviva, Amgen, Boehringer Ingelheim, Alcon, Aerpio, Kalvista, Ionis, Xplore, Mylan, Samsung, Novartis, Opthea, Chengdu Kanghong Biotechnology, Clearside Biomedical, Astellas, Allegro, Alimera Sciences, Ophthotech/IVERIC Bio, Outlook, Gemini Therapeutics, Genentech, ThromboGenics, Tyrogenex, Graybug Vision, Topcon, Optos, Gyroscope Therapeutics, Stealth, Spiam, Aerie, Apellis, Roche, Novartis, OHR, Xplore, RegenxBio, Kodiak Sciences, Zeiss, Annexon, Oculis and Regeneron. |
Diabetic retinopathy remains the leading cause of vision loss in developed countries with vision-threatening complications, including diabetic macular edema, vitreous hemorrhage or tractional retinal detachment. Anti-VEGF injections have been established as the primary treatment for DME and an alternative or adjunct to panretinal photocoagulation for proliferative DR.1-5
Challenge of anti-VEGF therapy
 |
|
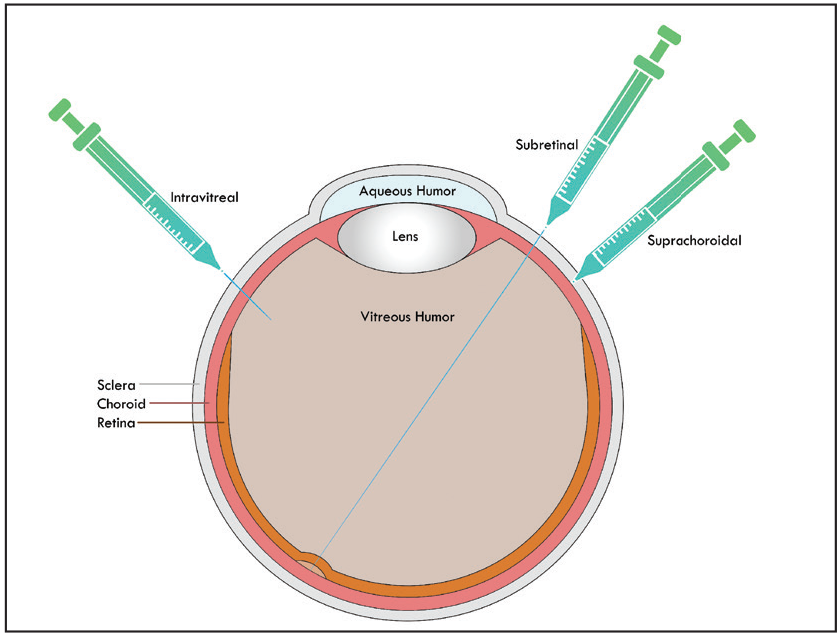
Figure 1. This illustration demonstrates the three delivery routes for retinal gene therapies: intravitreal injection; subretinal placement involving bleb formation; and suprachoroidal delivery. |
While intravitreal anti-VEGF treatment has been shown to be effective, it results in a significant injection burden, with patient compliance a significant barrier to optimal outcomes for proliferative DR and even as a first-line therapy for DME.6¬8 For PDR, PRP is most frequently done because clinicians often don’t consider anti-VEGF monotherapy due to the compliance, cost and treatment burden issues.
Recent PANORAMA and DRCR Retina Network Protocol W data establish the role of intravitreal aflibercept (Eylea, Regeneron Pharmaceuticals) in the improvement in scores in the Diabetic Retinopathy Severity Scale and reduction of vision-threatening complications—that is, DME and PDR—in eyes with severe non-proliferative DR.9 The retina community has also been reluctant to readily adopt anti-VEGF therapy for severe NPDR without DME as persistent/frequent injections are necessary for eyes that can be otherwise observed.
Gene therapy, allowing for the endogenous expression of anti-VEGF, has the potential to be an alternative to PRP in the treatment of PDR and to reduce injection burden in DME or in severe NPDR or PDR, as well as preventing vision-threatening complications while avoiding PRP-induced visual field loss and nyctalopia.
Gene therapy for retinal pathology
 |
|
Video 1. Allen Ho, MD, of Wills Eye Hospital, Philadelphia, demonstrates administration of subretinal RGX-314 in a patient with neovascular age-related macular degeneration. Available at: bit.ly/RetSpecMag_2022_01. (Courtesy Allen Ho, MD) |
The retina remains a unique target for gene therapy. In 2017, voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics/Roche) emerged as the first Food and Drug Administration-approved retinal gene therapy targeting the RPE65 mutation for retinal degeneration.10 This drug is delivered via subretinal injections of an adeno-associated virus (AAV) vector carrying a functioning copy of the RPE65 gene. Three-to-four-year follow-up data demonstrated sustained improvements in visual navigation, light sensitivity and visual field with a good safety profile.11
Gene therapy has also been explored for choroideremia and X-linked retinoschisis, with promising data employing subretinal vector administration.12,13
Gene therapy for DR and DME aims to provide an endogenous supply of
anti-VEGF that prevents disease progression (to DME, high-risk PDR and vitreous hemorrhages), reduces the number of supplemental anti-VEGF injections and alleviates treatment burden on patients.
Adverum’s preclinical assessments have demonstrated the potential of anti-VEGF gene therapy in providing safe and effective long-term treatment for DME and neovascular age-related macular degeneration.14 RegenxBio and Adverum are currently exploring the delivery of anti-VEGF gene fragments using AAV vectors to induce the endogenous production of anti-VEGF.
Delivery mechanisms
 |
Investigators are studying these three gene therapy delivery routes (Figure 1).
• Subretinal space. This has been the most widely evaluated route because it targets the retinal pigment epithelium and photoreceptors more so than other mechanisms, and it has the potential for safer immunologic outcomes compared to intravitreal delivery.15 However, subretinal approaches require surgical intervention with vitrectomy and come with the inherent drawbacks of adaptation, invasiveness, complications and cost.
• Intravitreal gene therapy. This in-office procedure is ideal because retina specialists are most familiar with it. Challenges, however, with intravitreal gene therapy include less targeted pharmacokinetic and drug delivery and reduced immune privilege.
• Suprachoroidal space. Defined as the potential space between the sclera and choroid, with a thickness of only 35 µm, it allows for an in-office administration of drug to the choroid, RPE and the neurosensory retina while bypassing the need for internal limiting membrane penetration required after intravitreal drug delivery.16
Suprachoroidal delivery is already employed in other pathologies. Suprachoroidal triamcinolone acetonide (Xipere, Clearside Biomedical) was approved by the FDA for uveitis-related macular edema.17 Additionally, suprachoroidal injections may provide greater drug bioavailability to the posterior pole with more diffuse gene expression.18 Suprachoroidal gene delivery may portend greater localized immune response compared to subretinal delivery, but likely results in less systemic humoral response compared to intravitreal delivery.19
Investigative gene therapies for diabetic retinal disease
 |
|
Video 2. In this video, Dennis Marcus, MD, administers suprachoroidal RGX-314 in a patient with diabetic macular edema. Available at: bit.ly/RetSpecMag_2022_02. |
• RGX-314 (RegenxBio). This candidate utilizes an AAV8 vector with a gene encoding an anti-VEGF antibody fragment. Phase I/IIa data evaluating the subretinal administration of RGX-314 in the treatment of nAMD has shown stable visual acuity, decreased central subfield thickness and reduced treatment burden compared with anti-VEGF injections (Video 1, page 17).20,21
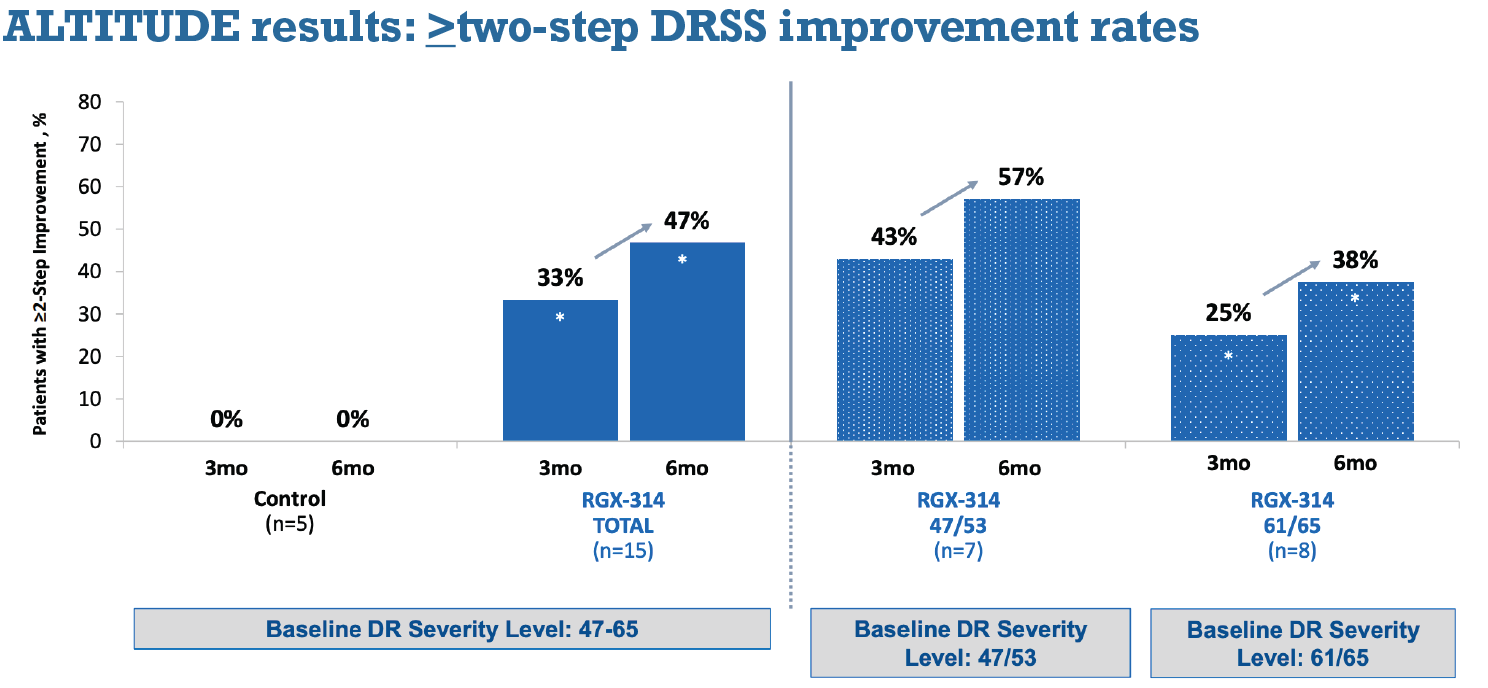
Early Phase II results from the ALTITUDE trial (NCT04567550) evaluating suprachoroidal RGX-314 for DR without center-involved DME demonstrated improvement in DRSS level, minimal adverse effects and no cases of endophthalmitis or intraocular inflammation. No prophylactic topical, periocular or systemic steroids were administered (Video 2).22 Six-month data revealed that three of 15 treated eyes demonstrated one-step improvement in DRSS and seven eyes had more than a two-step improvement (Figure 2).
Greater-than-two-step DRSS improvement was observed in three of seven severe NPDR eyes and in two of eight PDR eyes, and increased to four of even and three of eight eyes, respectively, at six months. At six months, two-step improvement rates after suprachoroidal RGX-314 were comparable to those seen in severe NPDR eyes in the RIDE/RISE and PANORAMA trials.23,24
 |
|
Figure 2. Six-month data from the ALTITUDE trial demonstrated that the number of patients in Cohort 1 who had greater than two-step improvements in Diabetic Retinopathy Severity Scale score after treatment with RGX-314 increased from three to six months. (Courtesy RegnexBio) |
 |
Suprachoroidal delivery of RGX-314 for nAMD is also being studied in the AAVIATE trial (NCT04514653). Early Phase II data is promising and has shown stable visual acuity, CST and a reduced treatment burden over six months compared to a ranibizumab control group.25
• ADVM-022 (Adverum). Similar to RGX314, ADVM-022 (AAV.7m8-aflibercept) aims to reduce the treatment burden of DME through endogenous anti-VEGF production. The INFINITY trial (NTC04418427) is evaluating the safety and efficacy of intravitreal ADVM-022 in patients with DME. While the initial results were promising, patients in the high-genomic load group experienced significant intraocular inflammation, with three eyes developing hypotony that required surgical intervention.26
Study enrollment was terminated to further evaluate the safety profile. The etiology of this inflammation hasn’t been determined, although inadequate anti-inflammatory prophylaxis for high-dose treatment, comorbidities in affected patients, and diabetic- and vascular-related complications leading to the breakdown of the blood-retina barrier have been proposed.27,28
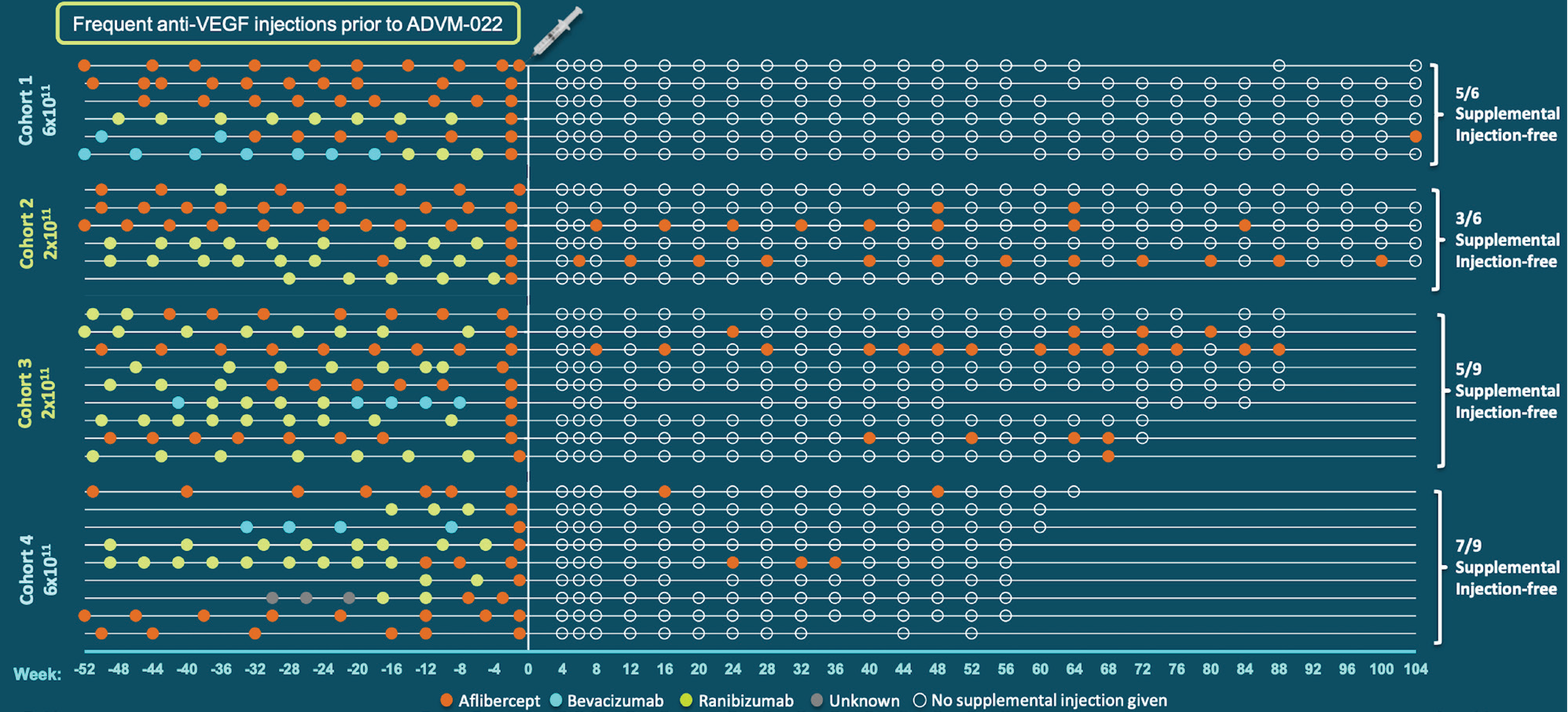
However, the low-dose treatment was well tolerated with no complications, mild-to-moderate inflammation and no cases of hypotony. Despite these complications, both high and low-dose ADVM-022 showed a greater probability of remaining free of rescue anti-VEGF injections over a 40-week period compared to the aflibercept controls (Figure 3).
 |
|
Figure 3. This chart shows that the majority of patients treated with ADVM-022 didn’t require supplemental anti-VEGF treatment. The white line indicates treatment with ADVM-022, with the plot to the left indicating pretreatment anti-VEGF injections, and the plot to the right indicating posttreatment injections. The far left column shows the size of the dose in vector genomes per eye given in each cohort. (Courtesy Adverum) |
Intravitreal ADVM-022 for nAMD also may be promising. Two-year OPTIC data (NCT03748784) demonstrated an 80-percent reduction of annualized injection frequency over two years, while maintaining a stable to improved CST and stable visual acuity of low dose intravitreal ADVM-022 compared to aflibercept control.29,30
Bottom line
Anti-VEGF gene therapy for DR with and without DME remains a potential exciting “one-and-done” approach in a patient population known for noncompliance. Early data with anti-VEGF gene therapy for DR and nAMD show stable visual acuities and reduced treatment burden. Regression of DR in some eyes indicates objective anatomic improvement using office-based suprachoroidal injection.
These encouraging short-term studies must be balanced by the potential of gene therapy-induced inflammation and autoimmune response and other potential safety concerns, as evidenced already in the INFINITY trial for DME with intravitreal ADVM-022. Further study of optimal techniques and delivery approaches as well as vector type will further elucidate the promise of anti-VEGF gene therapy for our diabetic patients. RS
REFERENCES
1. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and Vision. 2015;2:17.
2. Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two Phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022.
3. Nguyen CL, Lindsay A, Wong E, Chilov M. Aflibercept for diabetic macular oedema: A Meta-analysis of randomized controlled trials. Int J Ophthalmol. 2018;11:1002-1008.
4. Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126:87-95.
5. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, Phase 2b, non-inferiority trial. The Lancet. 2017;389:2193-2203.
6. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA Ophthalmol. 2018;136:1138-1148.
7. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmology Retina. 2018; 2:1179-1187.
8. Obeid A, Gao X, Ali FS, ET AL. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF Injections. Ophthalmology. 2018; 125:1386-1392.
9. Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti–vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: The Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139:701-712.
10. FDA approves hereditary blindness gene therapy. Nat Biotechnol. 2018;36:6.
11. Maguire AM, Russell S, Chung DC, et al. Durability of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease: Phase 3 results at 3 and 4 Years. Ophthalmology. 2021; 128:1460-1468.
12. Abbouda A, Avogaro F, Moosajee M, Vingolo EM. Update on gene therapy clinical trials for choroideremia and potential experimental therapies. Medicina. 2021;57:64.
13. Cukras C, Wiley HE, Jeffrey BG, et al. Retinal AAV8RS1 gene therapy for X-Linked retinoschisis: Initial findings from a Phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26:2282-2294.
14. Gelfman CM, Grishanin R, Bender KO, et al. Comprehensive preclinical assessment of ADVM-022, an intravitreal anti-VEGF gene therapy for the treatment of neovascular AMD and diabetic macular edema. J Ocul Pharmacol Ther. 2021; 37:181-190.
15. Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58:217-226.
16. Emami-Naeini P, Yiu G. Medical and surgical applications for the suprachoroidal space. Int Ophthalmol Clin. 2019;59:195-207.
17. Bausch + Lomb and Clearside Biomedical announce FDA approval of Xipere (triamcinolone acetonide injectable suspension) for suprachoroidal use for the treatment of macular edema associated with uveitis. [Press release] Bausch Health Companies, Laval, Quebec; Clearside Biomedical, Alpheretta, Ga.; October 25, 2021. Available at: https://www.prnewswire.com/news-releases/bausch--lomb-and-clearside-biomedical-announce-fda-approval-of-xipere-triamcinolone-acetonide-injectable-suspension-for-suprachoroidal-use-for-the-treatment-of-macular-edema-associated-with-uveitis-301407260.html. Accessed February 28, 2022.
18. Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58-66.
19. Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev. 2020;16:179-191.
20. Guimaraes TACd, Georgiou M, Bainbridge JWB, Michaelides M: Gene therapy for neovascular age-related macular degeneration: rationale, clinical trials and future directions. Br J Ophthalmol 2021, 105:151-157.
21. RegenxBio announces additional positive interim Phase I/IIa and long-term follow-up data of RGX-314 for the treatment of wet AMD. [Press release] RegenxBio; Rockville, Md.; February 16, 2021. Available at: https://www.prnewswire.com/news-releases/regenxbio-announces-additional-positive-interim-phase-iiia-and-long-term-follow-up-data-of-rgx-314-for-the-treatment-of-wet-amd-301228344.html. Accessed February 28, 2022
22. Klufas M. Suprachoroidal delivery of RGX-314 for diabetic retinopathy without CI-DME: Results from the Phase II ALTITUDE study. Paper presented at Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition; February 12, 20221.
23. Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2:997-1009.
24. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946-955.
25. Avery RL. Two year results from the subretinal RGX-314 gene therapy Phase 1/2a study for the treatment of NAMD, and an update on suprachoroidal trials. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day; Las Vegas, Nev.; November 12, 2021.
26. Boyer DS. Encore clinical data from the INFINITY trial of ADVM-022 intravitreal gene therapy for DME. Paper presented at American Academy of Ophthalmology; November 15, 2021.
27. Chan YK, Dick AD, Hall SM, Langmann T, Scribner CL, Mansfield BC, for the Ocular Gene Therapy Inflammation Working Group. Inflammation in viral vector-mediated ocular gene therapy: A review and report from a workshop hosted by the Foundation Fighting Blindness, 9/2020. Transl Vis Sci Technol. 2021;10:3.
28. Dimitrova G, Kato S, Tamaki Y, et al. Choroidal circulation in diabetic patients. Eye. 2001; 15:602-607.
29. Khanani AM. ADVM intravitreal gene therapy for neovascular AMD—Phase 1 OPTIC study. Paper presented at Retina Society; Chicago, IL; October 1, 2021
30. Busbee B, Boyer DS, Khanani AM, et al. Phase 1 study of iuntravitreal gene therapy with ADVM-022 for neovascular AMD (OPTIC Trial). Invest Ophthalmol Vis Sci. 2021;62:352-352.



