Take-home Points
|
 |
|
Bio Dr. Oskouei is vice president, biosimilars, at Cardinal Health, a health-care company whose services include pharmaceuticals distribution, global medical and laboratory products manufacturing and distribution, and performance and data solutions for health-care facilities. She also serves on the board of advisers of the Centers for Biosimilars. DISCLOSURES: Dr. Oskouei is an employee of Cardinal Health. |
In September the Food and Drug Administration approved Byooviz (ranibizumab-nuna, Samsung Bioepis/Biogen), the first ophthalmology biosimilar referencing Lucentis (ranibizumab, Genentech/Roche) to treat retinal conditions including neovascular age-related macular degeneration. This landmark approval is anticipated to expand treatment options with lower-cost, high-quality therapies for the approximately 11 million Americans diagnosed with AMD.
While the Biden Administration has pushed for more biosimilar education and options to create competition and lower drug prices in the United States, some physicians remain cautious, with early market research indicating ophthalmologists may be among the most hesitant.
As Byooviz prepares to launch next summer, retina specialists and ophthalmologists will have more treatment options than ever before, including current on-label use of branded biologics, off-label use of bevacizumab (Avastin, Genentech/Roche), and biosimilars, as well as potential novel treatment options anticipated to enter the market. In preparation for these market advancements, here are five things to know as retina biosimilars come to market:
1. Biosimilars are safe and effective
A biosimilar is an FDA-approved biologic that is highly similar to, and as safe and effective as, an existing FDA-approved biologic, known as the reference product. Like generics, biosimilars are expected to produce the same clinical result as a reference product, but at a lower cost. However, unlike small-molecule generics that are manufactured from chemical compounds and identical to their reference product, biologics are large, complex molecules manufactured from living cells. Therefore, they can’t be identically replicated, hence the term “biosimilar.”
The data requirements for biosimilars differ from those for reference biologics, but this doesn’t mean that biosimilars have lower approval standards. The FDA requires biosimilars to meet rigorous approval standards, which means patients and healthcare professionals can be assured of the safety, efficacy, and purity of biosimilars just as they would the reference products.
Since the first biosimilar approval in the United States in 2015, there are now 31 FDA-approved biosimilars referencing 11 originator products. They span multiple therapeutic areas including oncology, rheumatology, diabetes and now ophthalmology. Twenty of the 31 approved biosimilars are officially on the market, with the remaining products pending launches primarily due to patent litigation settlements between the brand and biosimilar companies.
2. Biosimilars are approved via a separate regulatory pathway
Through the Biologics Price Competition and Innovation Act enacted in 2010, an abbreviated approval pathway (351k BLA) was established for biosimilars to offer patients lower-cost, high-quality products. The biosimilars approval pathway essentially created a paradigm shift as to how providers evaluate the products, given that the data package required for biosimilar approval differs from what’s traditionally required of originator biologics.
The goal of an originator biologic approval pathway (351a) is to establish standalone safety and efficacy, which results in the greatest regulatory weight being placed on the clinical studies. However, with the biosimilars approval pathway (351k), the greatest regulatory weight is put on the physiochemical characterization of the molecule since the goal of the pathway is to establish a high level of similarity with the originator biologic. This can result in a single, confirmatory clinical study conducted for the biosimilar in the most sensitive population to address any residual uncertainty after completing all other product analyses.
Once a high level of similarity is established with the originator biologic in terms of safety, efficacy and potency, extrapolation can occur. Extrapolation refers to the approval of indications held by the reference biologic without conducting additional clinical studies. Although the concept of extrapolation is not entirely new for biologics1–as it has been used for several years when originator biologics undergo manufacturing changes–the lack of familiarity with overall regulatory terms and the approval pathway associated with biosimilars can create hesitation among various stakeholders.
 |
3. Ophthalmic biosimilars have the potential to alleviate financial burden associated with retinal conditions
Latest estimates reveal that overall savings from biosimilars could exceed $100 billion by 2024.2 With an average discount range of 15 to 30 percent for the biosimilars thus far, these agents are expected to bring cost savings to some of the most common treatment options for retinal disorders, including AMD. PlatformQ Health has estimated that the U.S. economic burden from direct health-care costs due to AMD is $4.6 billion.3 Through increased competition, biosimilars have the potential to alleviate some of the financial burden associated with current anti-VEGF therapies, enhancing accessibility and affordability of these critical treatments for patients.
4. Biosimilars may be met with caution in ophthalmology
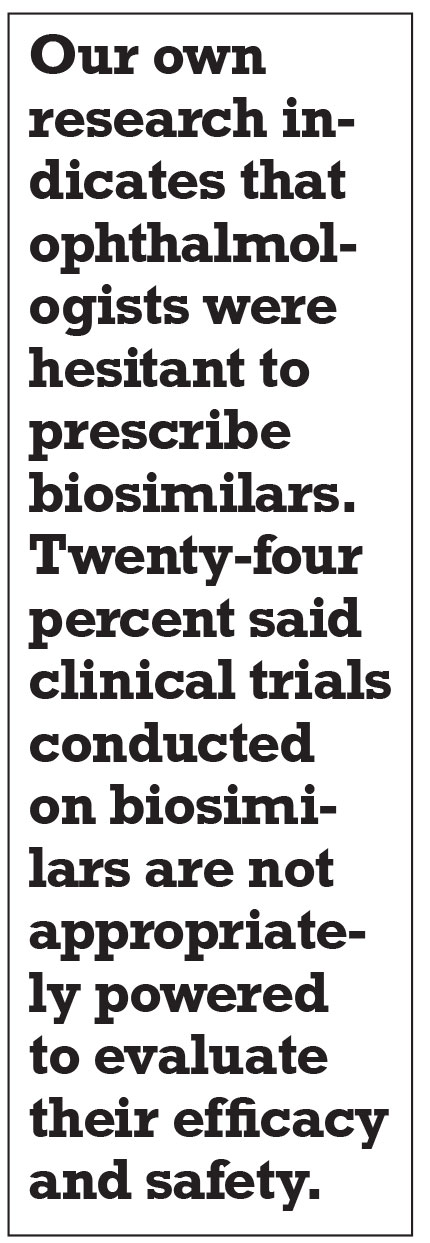
Our own research from early 2021 indicates that ophthalmologists were hesitant to prescribe biosimilars.4 Survey responses from 75 U.S. ophthalmologists revealed that 24 percent said clinical trials conducted on biosimilars are not appropriately powered to evaluate their efficacy and safety, and 35 percent have very limited knowledge of clinical trial design for biosimilars.
When asked about primary concerns with prescribing biosimilars once available, “uncomfortable from a clinical standpoint” and “payer coverage concerns” were equally rated as the top answers across all respondents. Both clinical and financial considerations were overall themes in the research, with the leading decision criteria for using an anti-VEGF biosimilar being “clinical studies and real-world evidence,” followed by “cost/price discount.”
 |
Another factor that may uniquely impact ophthalmology biosimilars is the lack of international real-world data. In oncology and rheumatology, biosimilars were approved and available in Europe well before the United States, meaning that U.S. providers could access more than 15 years of combined real-world data when the products were approved by the FDA. However, because the FDA approval of Byooviz is one of the first ophthalmic biosimilar approvals globally (following European approval weeks earlier), providers and other stakeholders won’t have the robust international data to reference. Rather, the United States has the opportunity to serve as leaders in this space and capture data and real-world evidence that can be referenced around the globe.
Additionally, the current prevalent off-label use of Avastin (bevacizumab, Genentech/Roche) is another consideration that will likely influence adoption decisions around the ranibizumab biosimilar. The intraocular use of compounded bevacizumab began while anti-VEGF drugs like ranibizumab were under development and has now been used for more than a decade.
Off-label bevacizumab’s safety and efficacy were shown in early studies and is now backed by several years of data. It’s one of the most commonly used anti-VEGF agents in AMD, and at a fraction of the cost of other treatment options. Therefore the pricing strategy (and the extent of discount), as well as payer contracts for the ranibizumab biosimilar will likely play a significant role in adoption.
Depending on the overall economic profile, the biosimilar could influence not only treatment decisions with the originator biologic, but also potentially current treatment decisions with compounded, off-label bevacizumab, other existing anti-VEGF agents, or even additional novel treatment options anticipated to come to market within the next year.
Overall, early insights gained with ophthalmologists on biosimilars are comparable to the market research findings with oncologists and rheumatologists prior to the first biosimilar approval in their respective areas. Past experiences can be leveraged to understand the types of considerations that go into adopting biosimilars, and proactive steps can be taken to address potential knowledge gaps before the launch of the first ophthalmic biosimilar.
 |
5. Providers educating themselves about biosimilars is critical
Building awareness and understanding among providers and other key stakeholders will be key to biosimilar adoption in ophthalmology. As our research earlier this year revealed,4 most providers lack familiarity and comfort with biosimilars, but there’s a strong desire for more education. In fact, 82 percent of survey respondents noted that educational information about safety, efficacy and performance would help them achieve a greater understanding of biosimilars.
It’s never too early to start the education process. By leveraging various resources to drive proactive educational efforts, providers will be able to evaluate biosimilars effectively and help champion the biosimilar education process moving forward.
The approval of the first ranibizumab biosimilar represents significant, and much needed, advancement in expanding treatment options for patients with debilitating retinal disorders.
Cost, payer coverage, clinical data, and education all play a critical role in the future use of biosimilars in ophthalmology. With the entrance of biosimilars and the potential approval of new innovator products, ophthalmologists will soon have a broader range of treatment options for delivering high-quality, affordable care to optimize patient outcomes. RS
REFERENCES
1. Weise M, Kurki P, Wolff-Holz E, Bielsky MC, Schneider CK. Biosimilars: The science of extrapolation. Blood. 2014;124:3191-3196.
2. The IQVIA Institute. Biosimilars in the United States 2020-2024: Competition, savings and sustainability. September 29, 2020. https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2020-2024.
3. Girgis L. Geographic atrophy and age-related macular degeneration. PlatformQ Health. https://www.platformqhealth.com/2017/07/13/geographic-atrophy-age-related-macular-degeneration/.
4. Oskouei ST. How lessons learned from oncology and rheumatology can pave the way for biosimilar success in ophthalmology. Paper presented at Festival of Biologics: World Biosimilar Congress USA. San Diego, Calif.; March 9 to 11, 2021.



