| Retina Standouts from ARVO |
|
Take-home Points
|
 |
|
Bio Dr. Abbey is a surgical and medical retina specialist at Texas Retina Associates, Dallas, and clinical assistant professor of ophthalmology at the University of Texas Southwestern Medical Center. DISCLOSURES: Dr. Abbey is a consultant to Allergan and Genentech/Roche. |
This year’s virtual ARVO 2021 meeting brought together the best researchers in retina to exhibit the latest in diagnostics, treatment, and management strategies for retinal disease. Every year, ARVO distinguishes itself from other ophthalmology meetings due to its inclusion of a broad spectrum of research—from innovative basic science to the latest clinical trials.
Here, we report on five compelling presentations in retina: results of DRCR.net Protocols AG, AH and W; the efficacy of half-dose photodynamic therapy vs. oral eplerenone for central serous chorioretinopathy; allogenic human retinal progenitor cells for retinitis pigmentosa; and intravitreal axitinib for neovascular age-related macular degeneration.
 |
DRCR.net Protocols AG and AH
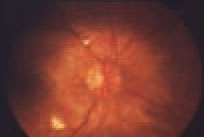
Previously published retrospective case series demonstrated the efficacy of in-office pneumatic vitreolysis (PVL) for symptomatic vitreomacular traction (VMT) and VMT with associated full-thickness macular hole (FTMH). DRCR Research Network Protocols AG (symptomatic VMT) and AH (VMT with associated small FTMH) were the first prospective trials to evaluate PVL in these eyes.
Protocol AG was a randomized, controlled trial that included eyes with VMT ≤3 mm in width on optical coherence tomography. It excluded eyes with lamellar or full-thickness macular holes. Best-corrected visual acuity ranged from 20/32 to 20/400. Forty-six eyes were randomized to receive either PVL (IVT injection of 0.3 mL of 100% C3F8) or sham injection. Patients were seen at one, four, 12 and 24 weeks post-injection. Rescue vitrectomy was recommended if their vision decreased from baseline by ≥5 letters at two consecutive visits (beginning at week 4) or by 10 letters or more from baseline at any visit.
Seventy-eight percent of PVL eyes achieved VMT release without the need for rescue vitrectomy, compared to just 9 percent of sham (p < 0.0001). At 24 weeks, BCVA didn’t significantly improve in the PVL group vs. sham. However, the PVL group did exhibit a significant reduction in loss of ellipsoid zone integrity (27 percent vs. 50 percent in sham, p < 0.009). One eye in each group developed a FTMH.
Protocol AH included eyes with VMT ≤3 mm in width on OCT and associated small FTMH ≤250 µm. BCVA ranged from 20/25 to 20/400. Mean macular hole diameter was 79 µm. Thirty-five eyes were randomized to receive either PVL (IVT injection of 0.3 mL of 100% C3F8 followed by four days of face-down positioning for half of each day) or sham injection. Patients were seen at one, four, 12 and 24 weeks postinjection. Rescue vitrectomy was recommended if the macular hole diameter didn’t improve after four weeks. Twenty-nine percent of eyes receiving PVL achieved macular hole closure, and 34 percent required rescue vitrectomy. Mean baseline visual acuity improved from 20/80 to 20/50 with PVL.
Unfortunately, PVL resulted in retinal tear or detachment in 12 of the treated eyes compared to none of the sham eyes. Due to these safety concerns, the steering committee decided to terminate these trials early.
Both studies demonstrated encouraging efficacy of PVL for the treatment of VMT and VMT-associated FTMH, but the risks of retinal tear and detachment appear to be unacceptable to most. PVL appears to be a somewhat effective treatment for VMT, but, because of its potential safety issues, it should be limited to patients with significant contraindications for surgery.
Lead author Clement K. Chan, MD, reports commercial relationships with Allergan/AbbVie, Amgen, Chengdu Kanghong Pharma, Iveric Bio, Regenerative Patch Technologies, Regeneron Pharmaceuticals and Genentech/Roche. Co-author Calvin Mein, MD, reports relationships with Apellis, Novartis, Regeneron and Amazon Smile.
 |
DRCR.net Protocol W
There has been considerable debate among retina specialists regarding the treatment of patients with moderate to severe nonproliferative diabetic retinopathy. These patients are at high risk for developing vision-threatening complications, and a recent trend has emerged toward early treatment of these patients with IVT anti-VEGF injections, even if they don’t have macular edema.
 |
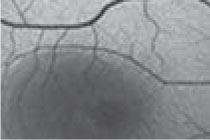
DRCR.net Protocol W aimed to determine if aflibercept (Eylea, Regeneron Pharmaceuticals) can prevent the development of PDR and center-involved diabetic macular edema over two years. It also aimed to determine if there was an associated visual benefit at four years.
This was a multicenter, prospective, randomized clinical trial in eyes with moderate to severe NPDR (ETDRS severity levels 43-53) without CI-DME. BCVA was 20/25 or better. Eyes were randomized to IVT aflibercept or sham at baseline, one, two and four months, followed by every four months through two years. For the next two years, the eyes would receive treatment only if the disease severity was worse than mild NPDR. Both groups received aflibercept with the development of PDR or CI-DME.
The study included 399 eyes: 200 in the aflibercept group and 199 in the sham group. At two years, the incidence of CIDME was 15 percent in the sham group and 4 percent in the aflibercept group (hazard ratio [HR] 0.36). The incidence of PDR was 33 percent and 14 percent in the groups, respectively, (HR 0.34). The combined incidence of PDR or CI-DME was 16 percent in the treatment group and 44 percent in sham (HR 0.32).
Although aflibercept reduced the incidence of vision-threatening complications, it didn’t result in a significant difference in BCVA at two years. Also, central subfield thickness (CST) on OCT wasn’t found to be significantly different at two years.
This study clearly establishes the efficacy of aflibercept for the improvement of DR. However, the lack of visual benefit at two years makes it difficult to justify this approach for most cases of moderate to severe NPDR at this time. Frequent monitoring with dilated exam, OCT and widefield angiography is critical to ensure optimal outcomes for these high-risk patients. If a visual benefit is proven with the pending four-year data, this treatment for NPDR will likely be more widely adopted.
Lead author Raj K. Maturi, MD, reported commercial relationships with Allergan/ AbbVie, Boehringer-Ingelheim, Genentech/ Roche and Unity Biotechnology.
 |
Oral eplerenone vs. PDT for CSCR
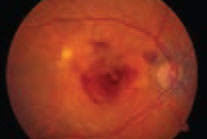
If SRF were present at the three-month follow-up, the patient would receive crossover treatment. In the primary oral eplerenone patients, 38 of 46 (82.6 percent) had persistent SRF and required crossover; 11 of 50 (22 percent) that received primary half-dose PDT required crossover. At the final follow-up visit 12 months after baseline, complete SRF resolution occurred in 38 of 39 (97.4 percent) patients with primary resolution after PDT; seven of seven in those with primary resolution after eplerenone; 30 of 35 (85.7 percent) in the eplerenone-to-PDT crossover group; and five of nine (55.5 percent) in the PDT-to-eplerenone crossover group. The researchers found no significant differences in BCVA and retinal sensitivity on microperimetry among the groups at the final visit.Due to a paucity of gold-standard evidence, consensus for treatment of chronic central serous chorioretinopathy (CSCR) remains lacking. The SPECTRA study was an open-label, multicenter, randomized controlled trial to compare the anatomic and functional efficacy and safety of primary treatment with either half-dose photodynamic therapy (PDT) or 25-mg oral eplerenone daily for a week, with or without crossover in patients with CSCR. Patients with fovea-involving subretinal fluid (SRF) persisting for more than six weeks and BCVA of 20/200 or better were randomized 1:1 to receive either half-dose PDT or oral eplerenone.
 |
This study demonstrated the superiority of half-dose PDT in the treatment of chronic CSCR, even after eplerenone treatment. CSCR remains one of the few indications for which I use PDT as a first-line therapy. It’s reassuring to see randomized controlled trial data supporting this decision. Crossover to eplerenone in patients that had suboptimal treatment with PDT wasn’t as likely to result in resolution of SRF. We need more data to determine the best treatment for this small subset of patients.
The study authors have no disclosures.
 |
Allogeneic human retinal progenitor cells for RP
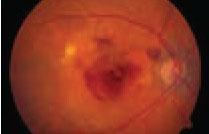
The use of stem cells and progenitor cells for a number of degenerative retinal conditions continues to advance. In this trial, patients with retinitis pigmentosa received an IVT injection of allogeneic human retinal progenitor cells (hRPC), which secrete neurotrophic factors that promote photoreceptor cell survival and function.
Eighty-four RP patients with BCVA of 20/80 to 20/800 were randomized to either a single injection of 3.0x106 hRPC, 6.0x106 hRPC or sham. Mean changes from baseline to month 12 were +2.81 letters in the sham group (n=26); +2.96 letters in the 3.0x106 hRPC group (n=25); and +7.43 letters in the 6.0x106 hRPC group (N=23).
A post hoc analysis was performed in a subgroup that had baseline central fixation, no severely constricted field (≥12 degrees in diameter) in the study eye, and no significant difference in BCVA between the study and fellow eye (≤15 letters). In this subgroup, mean BCVA changes from baseline to month 12 were +1.85 letters in the sham group (n=13); -0.15 letters in the 3.0x106 hRPC group (n=13); and +16.27 letters in the 6.0x106 hRPC group (n=11; p = 0.003 for 6.0x106 hRPC vs sham).
The 6.0x106 hRPC group also showed significant improvements in low-luminance mobility testing, contrast sensitivity, kinetic visual fields and visual function questionnaire VA LV VFQ-48 compared to sham. One treatment-related serious adverse event—ocular hypertension—was reported in the 3.0x106 hRPC group. It was treated with antihypertensive therapy.
The neuroprotection of hRPC therapy may preserve photoreceptor survival while restoring function to certain photoreceptors that haven’t fully degenerated. A primary advantage of this class of treatment is its ability to be gene agnostic; that is, it could theoretically be used for any of the mutations causing RP. The promising visual improvements and good safety profile in the 6.0x106 hRPC subgroup with better initial BCVA will pave the way for a much anticipated Phase III trial in the coming year.
Lead author David Liao, MD, reports a commercial relationship with jCyte.
 |
IVT axitinib implant for nAMD
Given the rapidly aging population, the need for greater durability to reduce the treatment burden for nAMD has become more pressing. OTX-TKI (Ocular Therapeutix), an IVT, bioresorbable, hydrogel-based implant, is designed to deliver the small-molecule tyrosine kinase inhibitor (TKI) axitinib in a sustained-release formulation to the vitreous, potentially extending treatment intervals in nAMD patients. TKIs have the potential for a broader anti-angiogenic profile than standard anti-VEGF agents.
This ongoing, prospective, Phase I study enrolled 20 patients with treatment-naïve or previously treated nAMD, separated into three treatment cohorts. IVT OTX-TKI was administered at doses of 200 µg (Cohort 1, n=6); 400 µg (Cohort 2, n=7); 600 µg (Cohort 3a, n=5/6); and 400 µg plus anti-VEGF induction therapy (Cohort 3b, n=2/6). Patients were assessed at days 0 (injection), 3, 7 and 14, followed by monthly assessments until the implants dissolved.
Preliminary results showed that BCVA and CST in Cohorts 1 and 2 remained relatively stable over the nine-to-12-month follow-up. Cohort 3 showed a sustained improvement in BCVA and CST over nine months of follow-up. In Cohorts 2 and 3, many eyes showed a decrease in CST and a clinically meaningful reduction in intraretinal and/or subretinal fluid by two months.
More than half of treated patients didn’t need additional supportive anti-VEGF treatment at six months. Several patients in Cohort 2 demonstrated durability of therapy for up to 12 months. The implant biodegraded in all subjects in Cohort 1 in nine to 10.5 months. There were no dose-limiting toxicities, ocular serious adverse events, or infections.
Currently available anti-VEGF therapies permit treatment intervals of up to three months in certain patients. OTX-TKI was able to maintain an acceptable level of disease activity in a large proportion of this cohort of nAMD patients for up to six months without additional treatment. Of course, further study is warranted.
Lead author James Wong, MD, reports a commercial relationship with Ocular Therapeutix. Co-authors report relationships with Ocular Therapeutix, Allegro, Allergan/AbbVie, Genentech/Roche, Graybug, Novartis, Optistent, Placid0, Pr3vent and Regeneron Pharmaceuticals. RS
REFERENCES
1. Chan CK, Mein CE, DRCR Retina Network. A randomized clinical trial of pneumatic vitreolysis with C3F8 for vitreomacular traction and a single-arm study of pneumatic vitreolysis with C3F8 for macular hole: DRCR Retina Network Protocols AG and AH. Invest Ophthalmol Vis Sci. 2021;62:3667.
2. Maturi R. A randomized trial of intravitreous anti-VEGF for prevention of vision threatening complications of diabetic retinopathy (Protocol W). Invest Ophthalmol Vis Sci. 2021;62:1041.
3. Feenstra H, van Rijssen TJ, Diederen R, et al. Long-term followup of chronic central serous chorioretinopathy patients after primary treatment of oral eplerenone or half-dose photodynamic therapy and crossover treatment: SPECTRA trial report No. 3. Invest Ophthalmol Vis Sci. 2021;62:2170.
4. Liao D, Boyer DS, Kaiser P, et al. Intravitreal injection of allogeneic human retinal progenitor cells (hRPC) for treatment of retinitis pigmentosa: A prospective randomized controlled Phase 2b trial. Invest Ophthalmol Vis Sci. 2021;62:3240.
5. Wong JG, Chang A, Guymer RH, et al. Phase 1 study of an intravitreal axitinib hydrogel-based implant for the treatment of neovascular age-related macular degeneration (nAMD). Invest Ophthalmol Vis Sci. 2021;62:218.



