 |
Retinal Vein Occlusion
Retinal vein occlusions (RVO) are the second most common cause of retinal vascular disease after diabetic retinopathy.1 Occlusions can involve the central retinal vein (CRVO) or hemicentral or branch retinal veins (BRVO). Though not completely understood, occlusions likely lead to a combination of increased venous pressure, production of cytokines such as interleukin-6 (IL-6), and production of vascular permeability factors including vascular endothelial growth factor (VEGF).2 These processes result in destruction of the blood-retinal barrier. Macular edema (ME) is just one of several devastating complications of RVO that can cause vision loss.
In the past, the Central Vein Occlusion Study recommended observation for ME in CRVO, and the Branch Vein Occlusion Study suggested grid laser therapy for ME in BRVO.3,4 However, advances in our understanding of disease pathology have led to new therapeutic options, the two main classes of which
 |
| Figure 1. The Ozurdex implant (Allergan) next to a dime for comparison (not sized to scale), and the 22-gauge applicator used to introduce the implant into the vitreous. |
Food and Drug Administration-approved anti-VEGF therapies for ME in RVO include ranibizumab (Lucentis, Genentech) and aflibercept (Eylea, Regeneron) with bevacizumab (Avastin, Genentech) used off label. These treatments target the ischemic component of RVO disease pathology. Trials have also shown steroids to reduce blood-retinal barrier breakdown by inhibiting production of factors like IL-6, VEGF and prostaglandins.5
A Paradigm Shift
A paradigm shift in RVO management occurred with the National Eye Institute’s Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) study. This prospective, randomized trial investigated CRVO and BRVO treated with the standard of care (observation and laser, respectively) vs. intravitreal injection of triamcinolone acetonide (TA) for ME secondary to RVO.6,7
The results of the BRVO arm demonstrated no significant differences between the sham and treatment arms in ≥15-letter improvement at 12 months.6 However, the CRVO arm showed a significant increase in subjects gaining ≥15 letters from baseline at 12 months in the 1-mg and 4-mg TA arms compared to the standard-of-care group (26.5 percent and 25.6 percent vs. 6.8 percent, respectively).7
These findings first suggested the utility of steroids in ME due to RVO, signaling a departure from the observational approach to CRVO. TA is commercially available as compounded TA, kenalog and Triesence (Alcon). Intravitreal use of kenalog and compounded TA is off-label, while Triesence and Trivaris (Allergan) are FDA-approved for intraocular use but not specifically for RVO. Although Trivaris was used in the SCORE studies, it is not commercially available.
Drug-delivery methods have also made significant progress. Anti-VEGF agents can be burdensome, oftenrequiring injections every month. Ozurdex (Allergan) is an extended-release dexamethasone-copolymer complex (Figure 1). Dexamethasone (DEX) is three times more potent than TA, but its half-life in the eye is less than six hours.2 However, in a polylactic-coglycolic acid polymer like Ozurdex, dexamethasone can persist in the eye for up to six months.
 |
The Ozurdex implant is injected into the pars plana with a 22-gauge needle. Ozurdex was FDA-approved in 2009, the first pharmacologic agent specifically approved for ME secondary to RVO.
The GENEVA studies demonstrated the efficacy of Ozurdex in treating ME secondary to RVOs.8 The studies consisted of two parallel, randomized controlled trials. A total of 1,256 patients were randomized into 0.7-mg and 0.35-mg DEX implant groups and a sham group; 66 percent had BRVO and 34 percent had CRVO. Eyes receiving either DEX dose showed 15-letter improvement in visual acuity significantly faster than the sham group (p<0.001). By six months, 41 percent of the 0.7-mg group and 40 percent of the 0.35-mg group had responded with at least a 15-letter gain compared with 23 percent in the sham group.
At day 180, 22 percent of the 0.7-mg DEX group still maintained at least 15-letter improvement, but this did not significantly differ from the sham arm. However, when the six-month analysis excluded patients with visits after day 180, the difference between the 0.7-mg and sham groups was significant (26.4 percent vs. 17 percent, p=0.017).8 Ozurdex is only expected to last six months, which may explain the significance attained by excluding visits after day 180 from the analysis.
GENEVA Study IOP Findings
A concern among physicians when using steroids is increasing intraocular pressure. While the treatment groups in the GENEVA study exhibited significantly more ocular hypertension (p≤0.002), the rise was responsive to topical IOP-lowering therapy in the majority of cases. Only five eyes from the DEX treatment groups required surgery to treat IOP. The trial noted no significant differences in IOP between the treatment arms and sham at six months.8
The GENEVA studies also
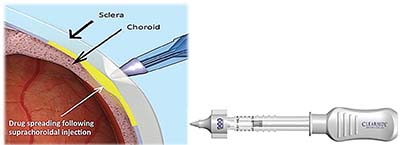 |
| Figure 2. CLS-TA (Clearside Biomedical) involves injection of the agent through a suprachoroidal approach (left). The CLS-TA applicator consists of a 30-gauge needle approximately 1,000 μm in length. |
Importantly, 21 percent of BRVO and 17 percent of CRVO patients only needed one implant over a year. Additionally, the safety profile of Ozurdex was similar after the second injection except with respect to cataract formation: 29.8 percent of phakic eyes treated with two 0.7-mg doses developed cataracts while only 10.5 percent of the delayed-treatment group did (p<0.001).9
Tailor Therapy Pending Trials
With the availability of both steroids and anti-VEGF agents for ME, retina specialists must decide which therapy to employ. As of yet, there are no published results from randomized controlled trials comparing anti-VEGF agents directly with corticosteroids for RVO.
Trials also need to evaluate combination therapies because future treatment will consist of regimens of both anti-VEGF agents and corticosteroids for many patients. The TANZANITE trial is the first FDA registration trial in RVO investigating combination therapy.10 This Phase II study examined combination therapy with CLS-TA (Clearside Biomedical), a formulation of TA injected suprachoroidally, with intravitreal aflibercept compared to aflibercept alone in ME secondary to RVO (Figure 2).
Early results showed the combination arm needed 60 percent fewer aflibercept injections after initial treatments compared to aflibercept monotherapy (nine vs. 23 injections, respectively, p=0.013). The Phase III study, Suprachoroidal Injection of Triamcinolone Acetonide with IVT Aflibercept in Subjects with Macular Edema Following RVO (SAPPHIRE), is underway.11
Until further evidence emerges regarding optimal therapy for ME in RVO, retina specialists must consider the needs of the individual patient. Generally, anti-VEGF treatments are administered first because they lead to quick recovery of vision.
Reassess Treatment Response
Physicians should reassess and react to patients’ responses to treatment. In the BRAVO trial of ranibizumab in BRVO, approximately 80 percent of the first-year visual acuity gains and a great majority of the reduction in macular thickness occurred by the third or fourth anti-VEGF injection.12,13 If by that time the patient shows no improvement, you must consider a different approach.
Partial responders show suboptimal responses in visual acuity gains and persistent ME after monthly anti-VEGF injections for three months. These patients are candidates for combination therapy with Ozurdex alongside the anti-VEGF agent.
Nonresponders who show no improvement from baseline VA, central retinal thickness (CRT) and macular morphology on optical coherence tomography after three months of anti-VEGF injections should start a different anti-VEGF agent, a steroid or a combination of the two.
We are
 |
| Figure 3. The Iluvien implant (Alimera Sciences) pictured next to a quarter for comparison (not sized to scale) and the 25-gauge applicator used to introduce the implant into the vitreous. |
Lastly, the patient’s ability to tolerate the treatment burden is critical. Anti-VEGF treatments often require monthly injections, which can be difficult for patients, especially if they’re receiving treatment in both eyes. Meanwhile, corticosteroid implants can be effective for several months. Thus, the treatment algorithm must also factor in the patient’s ability to adhere to the treatment course to achieving maximal outcomes.
Diabetic Macular Edema
Diabetic retinopathy (DR) is one of the leading causes of blindness worldwide. Vision loss associated with DR is most often due to diabetic macular edema (DME).14 Capillary leakage, fluid accumulation, and macular thickening occur alongside the breakdown of the blood-retinal barrier. This deterioration leads to the expression of inflammatory factors including vascular endothelial growth factor (VEGF), placental growth factor, and IL-6 and leukocyte migration into the retina.15,16 For decades, the standard of care for DME was medical management of diabetes along with focal/grid photocoagulation of leaking aneurysms and capillary beds.
The benefit of laser in DME was first shown in 1985 in the Early Treatment Diabetic Retinopathy Study (ETDRS).17 However, much progress has been made since in the development of pharmacological agents specifically targeting DME. In 2012, ranibizumab became the first FDA-approved pharmacologic treatment for DME, its benefits demonstrated in the RISE and RIDE trials.18 While anti-VEGF agents have been critical to the treatment of DME, the RISE and RIDE trials also showed that after two years of monthly injections, macular edema >250 μm central thickness on OCT persisted in 20 to 25 percent of patients.18 Additionally, approximately 40 percent of subjects did not achieve BCVA ≥20/40.18 Thus, while anti-VEGF agents have revolutionized the treatment of DME, the need for further pharmacologic treatments remains.
DRCR and Steroids for DME
The Diabetic Retinopathy Clinical Research (DRCR) Network Protocol I study was among the first randomized controlled studies to evaluate the use of steroids for DME.19 This study randomized eyes into four groups: sham also receiving prompt laser treatment; 0.5-mg ranibizumab group and prompt laser treatment; 0.5-mg ranibizumab with deferred laser treatment; or 4-mg TA with prompt laser. The primary outcome was visual acuity at one year.
The one-year mean change in letter score from baseline relative to sham was significantly greater in the ranibizumab-prompt laser and ranibizumab-deferred laser groups (+9 letters for each, p<0.001), but not in the TA-prompt laser group (+4, p=0.31) vs. the sham-prompt laser group (+3).
However, in a subanalysis of 273 pseudophakic eyes at baseline, visual acuity improvement in the TA-prompt laser group was comparable to that in the ranibizumab groups, suggesting that the visual acuity outcomes might have been influenced by cataract formation. This observation was supported by the fact that the TA-laser group, like the ranibizumab groups, exhibited statistically significant, decreased retinal thickening relative to sham.
While the results of the Protocol I study were not definitive regarding the use of TA in DME, the MEAD trial evaluating Ozurdex demonstrated the utility of steroids in DME.20 The MEAD study consisted of two randomized sham-controlled Phase III trials of 1,048 patients with DME. Patients were assigned to DEX 0.7-mg, DEX 0.35-mg and sham groups. Subjects were followed for three years and treated with Ozurdex no more than every six months.
The percentage of patients with ≥15-letter improvement in BCVA from baseline at year three or final visit was greater with DEX 0.7 mg (22.2 percent) and DEX 0.35 mg (18.4 percent) than with sham (12 percent; p≤ 0.018). Among phakic study eyes at baseline, the incidence of cataract-related adverse events was 67.9 percent, 64.1 percent and 20.4 percent (with 59.2 percent, 52.3 percent and 7.2 percent undergoing cataract surgery) in the DEX 0.7-mg, DEX 0.35-mg and sham groups, respectively.
Approximately one-third of patients in the DEX groups had significant increases in IOP requiring topical medication with returns to baseline by six months after each DEX implant. Only three patients in the DEX groups combined required trabeculectomy to manage IOP.20
Sustained-release FA
Another steroid studied for DME is fluocinolone acetonide (FA). Iluvien (Alimera Sciences) is a nondegradable sustained-release FA intravitreal insert introduced into the vitreous with a 25-gauge applicator (Figure 3). The FAME trial evaluated the use of Iluvien in DME. This was a parallel, three-year Phase III trial with 953 patients.21 The arms were a sham group, a 0.2-mg FA group and a 0.5-mg FA group. The percentage of patients with improvement of ≥15 letters from baseline at 24 months was 28.7 percent and 28.6 percent in the low- and high-dose FA groups, respectively, compared with 16.2 percent in the sham group (p=0.002 for each).
Among phakic eyes, the rates of cataract surgery were higher in the treatment arms with 74.9 percent of the low-dose group, 84.5 percent of the high-dose group and 23.1 percent of the sham group requiring cataract surgery. Incisional glaucoma surgery was performed in 8.1 percent of the high-dose group, 3.7 percent of the low-dose group and 0.5 percent of the sham group. We should note that the FDA label for Iluvien mitigates the risk of ocular hypertension by mandating that this implant be used only in DME patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in IOP. Adhering to the FDA label indications significantly reduces the risk of IOP rise after Iluvien administration.22
Iluvien is not to be confused with Retisert (Bausch + Lomb), an implant sutured to the anterior wall of the eye that releases FA into the anterior vitreous. Some investigators have drawn conclusions regarding the side-effect profile of Iluvien but referenced data from Retisert trials in noninfectious uveitis, for which it is FDA-approved, and not DME, for which Retisert is not approved.23 Studies of Retisert in DME found the insert caused significant reduction in edema, but about 20 percent of treated subjects required surgical treatment for elevated IOP at 24 months.24 Iluvien and Retisert differ both in their delivery and side-effect profiles and have not been evaluated head-to-head.
As with RVO, no randomized controlled trials have yet evaluated steroids and anti-VEGF agents head-to-head or in combination for DME. CLS-TA, as previously mentioned, is a TA formulation administered suprachoroidally. Phase I and II trials evaluating suprachoroidal CLS-TA with intravitreal aflibercept vs. suprachoroidal CLS-TA monotherapy for DME are under way.25 Until such studies are completed, retina specialists must tailor treatment to the individual patient.
When to Add Steroid Therapy
Anti-VEGF agents are undoubtedly first line for the vast majority of patients. However, as with RVO, the response to these agents requires constant re-evaluation. In the DRCR Protocol T trial, 34 percent of aflibercept subjects, 64 percent of bevacizumab subjects and 42 percent of ranibizumab subjects had >250 μm macular thickness at 12 months in spite of receiving 10 injections.26
In patients with partial responses at three months, retina specialists should consider the addition of a steroid such as Ozurdex or Iluvien. The patient’s comorbidities must also be factored in. A patient without glaucoma is a good candidate for earlier intervention with a steroid treatment. A patient with severe glaucoma who is not responding to an anti-VEGF agent may warrant a trial with a different anti-VEGF treatment before a steroid treatment is introduced. As with any medical treatment, understanding the broader medical and social context of the patient is critical to successful therapy.
In sum, the proliferation of treatment options for macular edema in RVO and diabetes has led to great improvements in outcomes. Many patients who would have otherwise been blinded are maintaining their vision. However, with an increasing number of therapies and little rigorous data comparing them as of yet, retina specialists are left to discern the best regimen for each patient.
Steroids offer a promising treatment class for macular edema that can be of great benefit to patients who do not respond optimally to anti-VEGF agents or cannot comply with the frequency of anti-VEGF treatment. Steroids come with their own side-effect profile, most notably increases in IOP and cataract formation. Retina specialists must weigh the consequences of these adverse events individually for each patient as their impact depends on patients’ underlying comorbidities. Ultimately, steroids are an effective adjunct in the treatment of macular edema from RVO and DME given the multifactorial nature of these diseases. RS
REFERENCES
1. Hellman JB, Fernandes JK, Patel RD, Hariprasad SM. Intravitreal dexamethasone implant plus prompt grid laser for macular edema due to retinal vein occlusion. Ophthalmol Res. 2014;2:325-335.
2. Hariprasad SM. Management of Retinal Vein Occlusion: Current Concepts. Thorofare, NJ: Slack Incorporated; 2014.
3. The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98:271-282.
4. Clarkson JG, Chuang E, Gass D, et al, for the Central Vein Occlusion Study Group M Report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102:1425-1433.
5. Wolfensberger TJ, Gregor ZJ. Macular edema—rationale for therapy. In: Coscas G, Cunha-Vaz J, Loewenstein A, Soubrane G, ed. Macular Edema: A Practical Approach. Vol 47. Basel: Karger; 2010:49-58.
6. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 6. Arch Ophthalmol. 2009;127:1115-1128.
7. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol. 2009;127:1101-1114.
8. Haller JA, Bandello F, Belfort R, Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134-1146.
9. Haller JA, Bandello F, Belfort R, Jr., et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453-2460.
10. Clearside Biomedical. Suprachoroidal injection of triamcinolone acetonide with IVT aflibercept in subjects with macular edema following RVO (TANZANITE). Available from: https://clinicaltrials.gov/ct2/show/NCT02303184. NLM identifier: NCT02303184. Accessed March 15, 2017.
11. Clearside Biomedical. Suprachoroidal injection of triamcinolone acetonide with IVT aflibercept in subjects with macular edema following RVO (SAPPHIRE). Available from: https://clinicaltrials.gov/ct2/show/NCT02980874. NLM identifier: NCT02980874. Accessed March 15, 2017.
12. Dugel PU, Hariprasad SM, Kitchens JW, Singer MA. A comprehensive approach to treating retinal vein occlusion. Retinal Physician. 2013:10(4):s6-18.
13. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion. Six-month primary end point results of a Phase III Study. Ophthalmology. 2010;117:1102-1112.
14. Hariprasad SM, Hunter A, Telander D. The evolving paradigm for the treatment of diabetic macular edema. Ophthalm Surg Lasers Imag Retina. 2013;44:324-328.
15. Hariprasad SM. Current Approaches to the management of diabetic macular edema. Am J Man Care. 2016:S292-S299.
16. Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690-1696.
17. No authors listed. A randomized clinical trial of early panretinal photocoagulation for ischermic central vein occlusion. Ophthalmology. 1995;102:1434-1444.
18. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular ddema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801.
19. The Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.
20. Boyer DS, Yoon YH, Belfort Jr R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914.
21. Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626-635.
22. Salvatore S, Bailey C, Chakravarthy U, Lotery AJ, Menon G, Talks J. The impact of prior steroid therapy on safety outcomes following treatment with Iluvien (fluocinolone acetonide)—an analysis of intra-ocular outcomes (IOP) from UK electronic medical records. Paper presented at: Association for Research in Vision and Ophthalmology 2017. May 10, 2017; Baltimore, MD.
23. Chin EK, Almeida DRP, Velez G, et al. Ocular hypertension after intravitreal dexamethasone (Ozurdex) sustained-release implant. Retina. November 1 2016.[Epublished ahead of print].
24. Pearson P, Levy B; for the Fluocinolone Acetonide Implant Study Group. Fluocinolone acetonide intravitreal implant to treat diabetic macular edema: 2-year results of a multi-center clinical trial. Invest Ophthalmol Vis Sci. 2005;46:4673.
25. Clearside Biomedical. Suprachoroidal injection of CLS-TA alone or with aflibercept in subjects with diabetic macular edema (HULK). Available from: https://clinicaltrials.gov/ct2/show/results/NCT02944240. NLM identifier: NCT02944240. Accessed April 18, 2017.
26. The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.



