Take-home points
|
 |
|
Bios Prof. Nawrocka is a vitreoretinal surgeon at the Ophthalmic Clinic Jasne Blonia, Lodz, Poland. Dr. Zajac is an ophthalmology resident with a focus on retina. Prof. Nawrocki is a vitreoretinal surgeon and head of the Ophthalmic Clinic Jasne Blonia and inventor of the inverted internal limiting membrane flap technique. He has received 11 Rhett Buckler Awards from the American Society of Retina Specialists for his innovative surgical concepts. DISCLOSURES: The authors have no financial interest to disclose. |
Nowadays, the role of vitrectomy in diabetic macular edema needs to be reconsidered. Proper patient selection might improve functional results and offer another choice of treatment for patients who can’t always come in for their monthly anti-VEGF injections.
Diabetic retinopathy is the sixth most common cause of blindness in the world. DME, defined as a retinal thickening involving or approaching the center of the macula, represents the most common cause of vision loss in patients affected by diabetes mellitus. One-hundred million people around the world have already been diagnosed with DME secondary to diabetes and the numbers are expected to increase in the coming years.1
Glycemic, BP, lipid control crucial
Currently, even with the various DME treatments that exist, most physicians choose anti-VEGF injections as the primary treatment. We must emphasize that regardless of the chosen strategy, controlling glycemia, blood pressure and lipids is always crucial in these patients.
The Diabetes Control and Complications Trial (DCCT) showed that in patients with type 1 diabetes, monitoring of glucose (measurements four times a day) resulted in a decrease in the rate of development of any retinopathy by as much as 76 percent, as well as a 54 percent reduction in the progression of established retinopathy compared to the conventionally treated group (with one time measurement per day). In cases of severe retinopathy, more rigorous glucose control may not be enough to prevent disease progression.2
The current treatments for DME include intravitreal pharmacotherapy, vitrectomy and, historically, laser photocoagulation of the macula. Intravitreal anti-VEGF injections have emerged as the gold standard for DME treatment. The discovery of the key role of vascular endothelial growth factor in initiating neovascularization changed the therapeutic approach in DME.3
Anti-VEGF agents, as we know, cause a significant decrease in central retinal thickness and improve visual acuity by 14 letters on average. Moreover, these drugs decrease the level of DR. Greater efficacy of anti-VEGF injections compared with laser therapy and corticosteroids has been proven. However, the use of laser therapy and corticosteroids seems to be growing slightly, especially as adjunctive therapy in anti-VEGF nonresponders.5
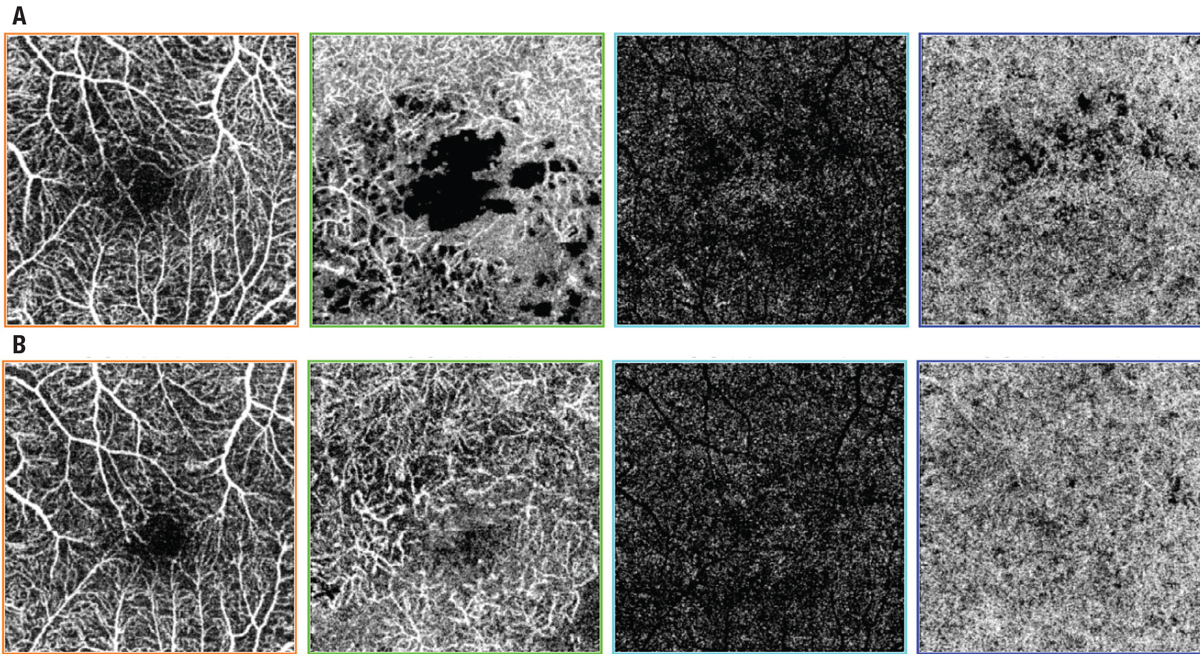 |
| Figure 1. Swept-source optical coherence tomography angiography scans of diabetic macular edema in a 66-year-old man A) preoperatively and B) one year after surgery. The scans depict, from left to right, superficial retinal vessels, deep retinal vessels, avascular zone and choroid, showing that the foveal avascular zone decreased after surgery in the superficial retinal vessels layer. |
Advantages of vitrectomy
Our group has suggested that vitrectomy also might play this role (Figure 1).4 We presented satisfactory results in treatment-naive patients.4 Not only did anatomy and vision improve, but we also managed to reduce to a minimum the frequency of future anti-VEGF injections. Thus, to achieve good results, proper patient selection seems to be crucial.
The disadvantages of anti-VEGF injections for DME are cost and frequency of administration. Intravitreal injection carries a higher risk of endophthalmitis in patients with diabetes than in patients with neovascular age-related macular edema. Moreover, anti-VEGF agents are teratogenic; thus, patients of child-bearing age must use contraception, and pregnant women can’t have them.
Controversy of surgery
The role of surgery in DME still remains controversial. Many studies have reported that anatomy improves, but visual acuity changes only variably.6,7 However, we must consider that one of the inclusion criteria for the DRCR Retina Network study was the surgeon’s determination that the selected patient had a poor prognosis to respond to further macular laser photocoagulation or had epiretinal membranes.6 That would suggest that only patients with long-standing macular edema qualified for surgery.
Studies have shown that previous photocoagulation can lead to worse outcomes after anti-VEGF treatment.2 So, we may extrapolate that laser photocoagulation might also contribute to worse visual outcomes in victrectomized eyes. It’s also clear that a long-lasting DME that responds poorly to treatment will result in lower final visual outcomes.
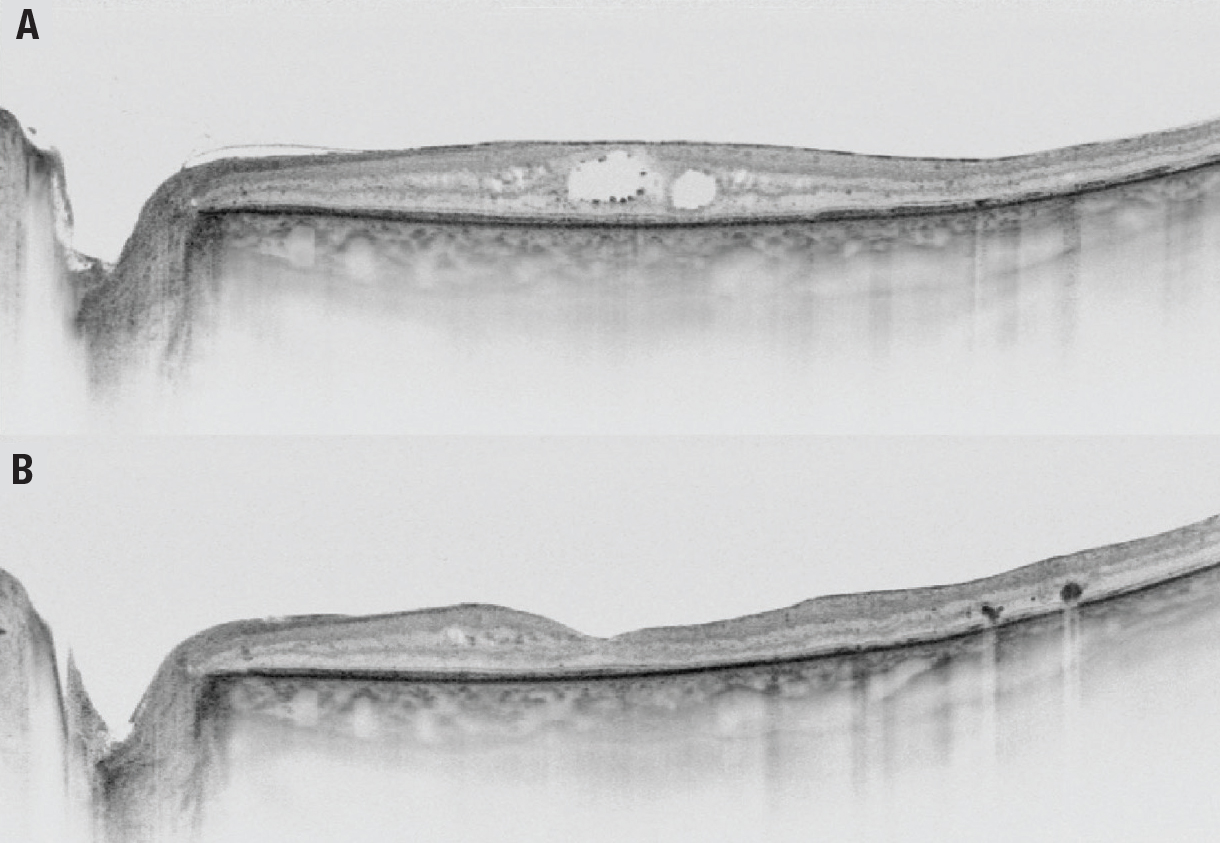 |
| Figure 2. Swept-source optical coherence tomography of diabetic macular edema in a 66-year-old man A) before surgery and B) one year after surgery. |
Proper patient selection for surgery is paramount (Figure 2). It has been reported that the best results after vitrectomy are obtained in patients with an intact external limiting membrane and ellipsoid zone.8 A study from Japan reported that patients with subretinal fluid in diffuse macular edema also benefit from surgery.9
ILM peeling
Several years ago, we published a study that reported that ILM peeling performed in the setting of complications of proliferative vitrectomy, such as vitreous hemorrhage and tractional retinal detachment, decreases the long-term incidence of macular edema.10
We also presented results of vitrectomy in treatment-naive DME patients.11 The study of 44 patients proved that both anatomical and functional results improve after vitrectomy with ILM peeling. We noted improvement of visual acuity of more than one line in 60 percent of eyes. Only three patients lost VA during the six-month observation period, mainly due to cataract formation (Video).
Even if anti-VEGF therapy is usually considered the first-line treatment, we observe initial visual gains three to six months after treatment starts.12 Similarly, after vitrectomy, improvements are expected within about four months.13
Predictors of poor outcome
With multivariate analysis, we identified three factors associated with poor final outcome:
- preoperative presence of ERM;
- duration of diabetes; and
- poor baseline visual acuity.
These data further explain why previous studies failed to show visual improvement after vitrectomy. Because most authors chose patients who were less likely to respond, the results were disappointing.
Recurrences of macular edema after vitrectomy are rare. We observed them in three eyes. In longer observation times (24 to 36 months), about 15 percent of our patients needed additional injections, according to our own unpublished data.
Because the viscosity of the vitreous is 300 to 2,000 times greater than the aqueous, earlier authors hypothesized that vitrectomy might reduce the half-life of anti-VEGF agents, but several studies proved the opposite.14,15 According to our own observation, these eyes usually respond to anti-VEGF therapy so that they don’t need multiple injections.
 |
Yuki Morizane, MD, PhD, and colleagues proposed subretinal balanced salt solution injections in the treatment of DME.16 Subretinal injections are usually recommended in subfoveal hemorrhage in nAMD, but in gene therapy they’re advised to be done paracentrally from the fovea. We need further studies to determine whether foveal detachment doesn’t cause any serious photoreceptor defects. Currently, we usually perform subretinal injections in DME with good results.17
Mechanisms favoring vitrectomy
Several mechanisms have been proposed in which vitrectomy might be helpful in the treatment of DME. Chronic inflammation, hypoxia and traction increase VEGF levels in tissues.18 The fact that the posterior hyaloid is usually attached in DME might trap VEGF and limit its extraretinal diffusion.19 The posterior hyaloid is usually thickened in DME and is more difficult to remove than it is during vitrectomies in other macular diseases. Also, the ILM is intraoperatively more fragile. It might not only trap VEGF, but it may also reduce the bioavailability of anti-VEGF agents in the retinal space.
Vitrectomy removes traction as well as glycation products and VEGF. Reducing vitreous viscosity increases the diffusion of molecules through the eye.20 This results in higher premacular oxygen concentrations and lower intraretinal VEGF concentrations. Vitreoretinal surgery with ILM peeling also induces the glial repairing processes.
Bottom line
Vitrectomy isn’t intended to be the first-line treatment for DME, but it should be considered for patients who live far from retinal clinics and aren’t able to come in for frequent injections or in patients with financial burdens. Moreover, vitrectomy might be discussed with pregnant women or women planning pregnancy. The obvious indications are the coexistence of traction or epiretinal membranes. Unfortunately, the disease is advanced in these eyes and final results aren’t always satisfactory. Good results after vitrectomy are expected in early-onset disease with subretinal fluid in patients without severe photoreceptor defects. RS
REFERENCES
1. Bandello F, Battaglia Parodi M, et al. Diabetic macular edema. Dev Ophthalmol. 2010;47:73-110.
2. No authors listed. Diabetes Control and Complications Trial (DCCT): Results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10:1-19.
3. Browning DJ, Stewart MW, Lee C. Diabetic macular edema: Evidence-based management. Ind J Ophthalmol. 2018;66:1736-1750.
4. Nawrocka Z. Nawrocki J. Vitrectomy in diabetic macular edema—A swept-source optical coherence tomography (OCT) angiography study. Ophthalmol Sci. In review.
5. He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018; 21;18:121.
6. Diabetic Retinopathy Clinical Research Network Writing Committee, Haller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 2010;117:1087-1093.e3.
7. Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: Diabetic Retinopathy Clinical Research Network. Retina. 2010;30:1488-1495.
8. Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415-1420.
9. Ichiyama Y, Sawada O, Mori T, Fujikawa M, Kawamura H, Ohji M. The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol. 2016:254:1545-1551.
10. Michalewska Z, Bednarski M, Michalewski J, Nawrocki J. The role of ILM peeling in vitreous surgery for proliferative diabetic retinopathy complications. Ophthalmic Surg Lasers Imaging Retina. 2013;44:238-242.
11. Michalewska Z, Stewart M, Landers MB, Bednarski M, Adelman RA, Nawrocki J. Vitrectomy in the management of diabetic macular edema in treatment naïve patients. Can J Ophthalmol. 2018;53:402-407.
12. Diabetic Retinopathy Clinical Research Network; Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2016: 26;372:1193-1203.
13. Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996:122:258-260.
14. Diabetic Retinopathy Clinical Research Network; Bressler SB, Melia M, Glassman AR, et al. Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 2015:35:2516-2528.
15. Michalewska Z, Nawrocki J. Anti-VEGF treatment might be continued even after vitrectomy for endophthalmitis in neovascular AMD. J Vitreoret Dis. 2020:4;6-10.
16. Morizane Y, Kimura S, Hosokawa M, et al. Planned foveal detachment technique for the resolution of diffuse diabetic macular edema. Jpn J Ophthalmol. 2015;59:279-287.
17. Nawrocka ZA, Nawrocka Z, Nawrocki J. Vitrectomy with ILM peeling in diabetic macular edema in one eye vs. intravitreal anti-VEGF injections in the second eye: A comparative study. Graefes Arch Clin Exp Ophthalmol. In review.
18. Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;16;49:634-646.
19. Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: The role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001:132:369-377.
20. Gisladottir S, Loftsson T, Stefansson E. Diffusion characteristics of vitreous humor and saline solution follow the Stokes Einstein equation. Graefes Arch Clin Exp Ophthalmol. 2009: 247:1677-1684.



