More evidence has emerged that deep learning can play a role in predicting progression from diabetic retinopathy to diabetic macular edema, with publication of a study that found a model using color fundus photographs and a relatively smaller dataset could predict changes in macular thickness detectable with time-domain optical coherence tomography.
Researchers from Roche in Switzerland and its Genentech unit in San Francisco reported in the journal Investigative Ophthalmology & Visual Science on a deep-learning (DL) model that used fundus photographs to quantify central subfield thickness (CST) and central foveal thickness (CFT), which correlated with measurements on TD-OCT.1
CST models most promising
The study evaluated four DL models, finding that the models using CST seemed to perform better than those using CFT. “This is likely due to the fact that the performance of a DL regression is generally more sensitive to the stability of the endpoint and the CFT is a less reliable endpoint compared to CST,” co-author Jeffrey R. Willis, MD, PhD, associate medical director of ophthalmology, at Genentech, tells Retina Specialist. “The binary classification models, upon further development with larger datasets, will be more likely to reach a performance high enough to become a diagnostic solution to be deployed in the real world.”
The best DL model was able to predict CST ≥ 250 μm and CST ≥ 400 μm with an area under the curve (AUC) of 0.97 (87.5-percent sensitivity, 96.4-percent specificity) and of 0.94 (99-percent sensitivity, 94.4-percent specificity), respectively. To predict CFT ≥ 250 μm and CFT ≥ 400 μm, the best DL model had an AUC of 0.91 (80-percent sensitivity, 85-percent specificity) and of 0.97 (90-percent sensitivity, 94-percent specificity), respectively.
Quality fundus photos key
The study found the quality of fundus photography can be a key factor in building predictive DL models. “The study told us that the performance of DL algorithms improves as the quality of the color fundus photographs improves,” says Dr. Willis.
“The idea here is that our proof-of-concept study showed that DL is able to quantify three-dimensional features (i.e., OCT measure of macular thickening [MT]) from a two-dimensional color fundus photograph,” he says. That 3-D quantification of MT can provide clues about the severity of DME.
Potential for smaller datasets
The study also showed the promise of using a relatively small dataset to construct DL models, as it used 18,000 fundus photographs and associated OCT measurements from the RIDE and RISE trials. “The benefit of using RIDE/RISE clinical trial data is that it's likely more standardized and perhaps of better quality than data taken in the real-world setting,” Dr. Willis says. “Our study also showed that a smaller dataset could be used to train DL algorithms if one takes a transfer-learning cascade approach”—that is, using learnings from other datasets before training the data on the final, smaller dataset.
The next step for the research team is to further train the model and validate it in real-world practice, Dr. Willis says.
| IN BRIEF Apellis Pharmaceuticals resumed enrollment in two Phase III trials, DERBY and OAKS, of intravitreal APL-2 for geographic atrophy. The company voluntarily paused enrollment last year because of reports of ocular inflammation in patients dosed with one manufactured batch. Enrollment should be completed by the end of the first quarter of 2020. Faricimab (Roche/Genentech) bispecific antibody is the subject of two large global Phase III clinical trials in wet age-related macular |
Ocular Therapeutix dosed the first patient in a Phase I trial of OTX-TKI (tyrosine kinase inhibitor implant) for wet age-related macular degener- ation at the Sydney Retina Clinic Australia. The Food and Drug Administration granted orphan drug designation for OCU400 (Ocugen), a novel gene therapy for the treatment of NR2E3 mutation-associated retinal degenerative disease. |
REFERENCE
1. Arcadu F, Benmansour F, Maunz A, et al. Deep learning predicts OCT measures of diabetic macular thickening from color fundus photographs. Invest Ophthalmol Vis Sci. 2019;60:852-857
No is the latest word on anti-VEGF and CV events
The evidence linking intravitreal anti-VEGF therapy with a heightened risk of cardiovascular events has been split, but a population-based, retrospective cohort study in JAMA Ophthalmology has reported that intravitreal anti-VEGF treatment for neovascular, or exudative, age-related macular degeneration has come down on the side that these treatments do not raise CV event risk.1
“Our treatment cohort, who received anti-VEGF therapy for e-AMD according to real-world practice patterns, was compared to an age-matched, e-AMD group prior to the availability of anti-VEGF treatment,” lead author Raymond Iezzi, MD, of Mayo Clinic in Rochester, Minn., tells Retina Specialist. Anti-VEGF drugs “introduced no statistically-significant increase in five-year risk of cardiovascular events in patients with e-AMD.”
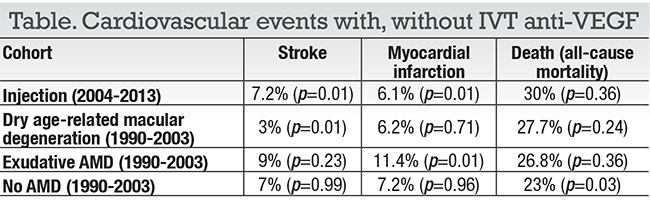
The study included 504 patients who had at least one anti-VEGF injection for e-AMD from 2004 through 2013 and matched to three cohorts from 13 previous years, when anti-VEGF treatments were not available: with e-AMD; with dry AMD; and controls with no history of AMD (Table). Most patients in the anti-VEGF group (n=377, 75 percent) received bevacizumab (Avastin, Roche/Genentech), and 292 patients received at least three IVT injections in the last year before having a CV event.
The anti-VEGF era cohorts had a five-year risk for stroke of 7.2 percent, for myocardial infarction (MI) of 6.1 percent and death of 30 percent. The study found, on multivariate analysis, a 63 percent increased risk of death compared with controls with e-AMD in pre-anti-VEGF era, but not the other control groups on multivariate analysis (p < 0.001).
“We found that while anti-VEGF-treated e-AMD patients had an increased five-year mortality risk compared to e-AMD patients who did not receive anti-VEGF therapy, no increased risk of mortality or cardiovascular events was noted when compared to the two other control groups,” he says. “Our overall conclusion is that anti-VEGF treatment did not increase the five-year risk of stroke, myocardial infarction or death in our population.”
The study called for additional research to evaluate CV risks in patients with dry AMD receiving anti-VEGF injections, as well as among different anti-VEGF agents.
 |
REFERENCE
1. Dalvin LA, Starr MR, AbouChehade JE, et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophtalmol. 2019 January 31. [epub before print]
2. Meyerhardt JA, Li L, Sanoff HK, Carpenter W 4th, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:608-615.



