 |
 |
 |
 |
Treatment strategies for diabetic retinopathy can vary based on disease severity. Some patients may need prompt treatment. Others can be safely observed depending upon their ability to comply with regular and frequent follow-up eye examinations.
Current first-line treatments for proliferative DR and diabetic macular edema are laser and intravitreal injection of both approved and off-label drugs (anti-VEGF, dexamethasone and fluocinolone acetonide of the former; bevacizumab and triamcinolone acetonide of the latter).1,2
Ongoing clinical trials and previous landmark study results provide important insights that help retina specialists determine the most effective management strategies. Here, we discuss outcomes of several important clinical trials.
Scope of the problem
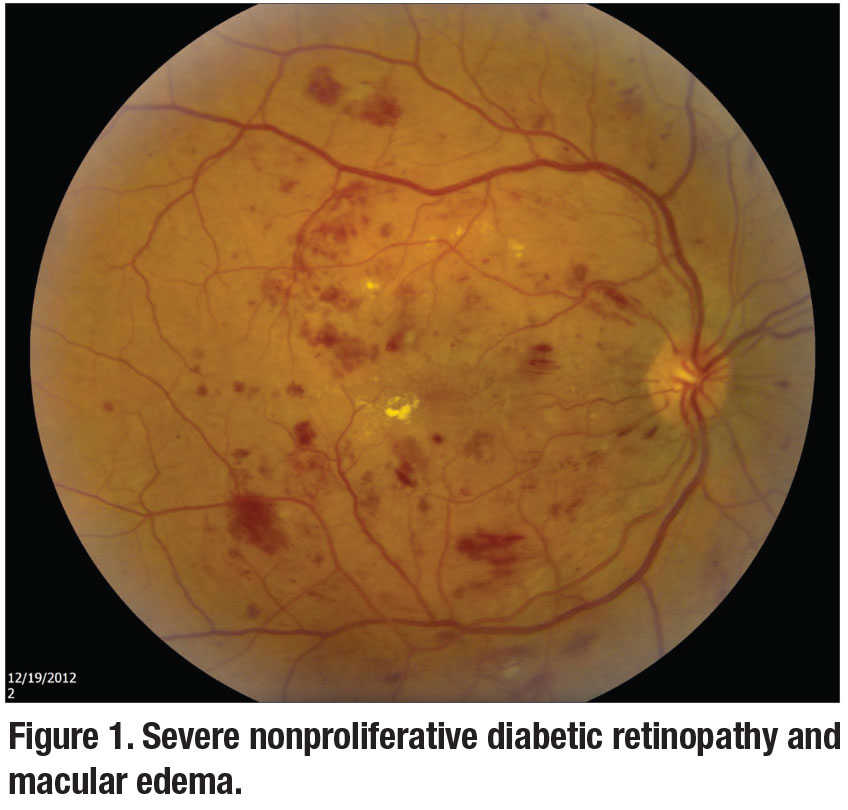
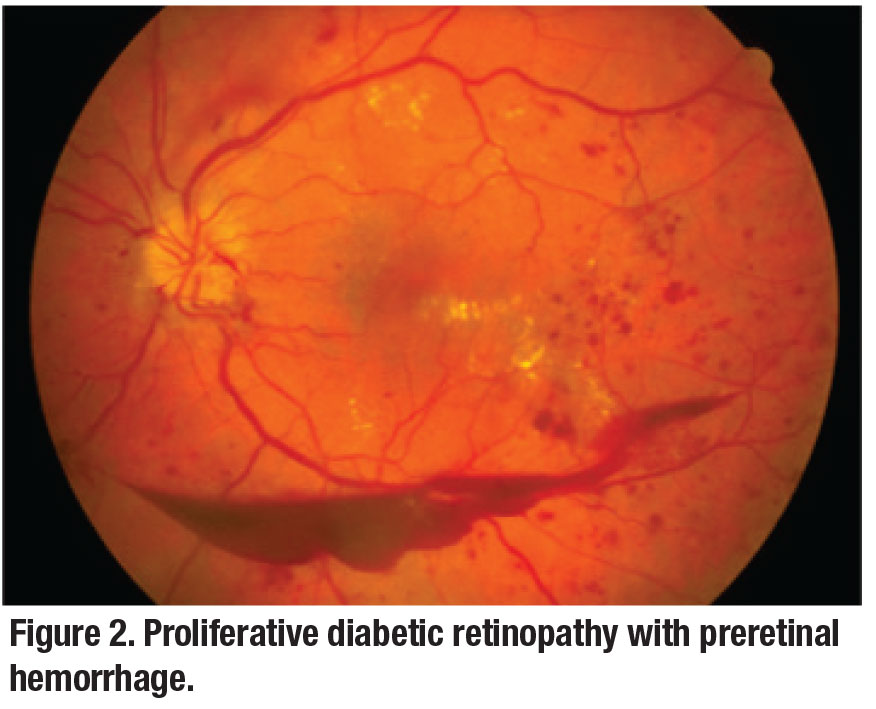
The Centers for Disease Control and Prevention reports that 26.9 million people of all ages—8.2 percent of the U.S. population—have been diagnosed with diabetes mellitus, 90 to 95 percent of whom are classified as having type 2 diabetes.3 DR (including DME and proliferative DR, Figures 1 and 2) is one of the most common end-organ manifestations of diabetes.4
Take homes from PANORAMA
It’s well known that patients with nonproliferative DR are at risk of progressing to vision-threatening events, including PDR and DME. The Phase III prospective, multicenter, randomized, controlled PANORAMA trial evaluated intravitreal aflibercept injections (IAI; Eylea, Regeneron Pharmaceuticals) vs. observation for patients with moderately severe to severe NPDR.5
This double-masked study enrolled 402 patients with NPDR (Diabetic Retinopathy Severity Scale [DRSS] level 47 and 53 confirmed by the central reading center), in whom panretinal photocoagulation could be safely deferred for up to six months. The study had three groups: sham; group 1, IAI q16 weeks; and group 2, IAI q8 weeks. All IAI patients received monthly loading doses for three to five months. The sham group didn’t receive IAI loading doses, although group 1 received three initial monthly doses as well as one q8-week interval dose, while group 2 received five monthly doses at the start of treatment. During the second year, group 1 (q16 weeks) continued to receive q16-week IAI, although group 2 (q8 weeks) received pro re nata q8-week IAI.
 |
At two years of follow-up, the percentage of patients with a two‑step or greater improvement on DRSS score from baseline (the primary study endpoint) was significantly higher in the IAI groups (50 and 60.2 percent in groups 1 and 2, respectively, vs. 12.8 percent). The trial confirmed that moderately severe to severe NPDR has significant risk of progressing to vision-threatening complications (VTC), including PDR, anterior segment neovascularization (ASNV) and center-involving DME (ci-DME).
The analysis showed a larger number of sham patients (30.6 percent) compared to IAI groups (9.1 and 6.9 percent in groups 1 and 2, respectively) developed VTC at two years. These complications tended to occur more frequently in patients with higher baseline DRSS scores.
The study results are encouraging, but patient compliance and regular follow-ups for multiple injections—at least six to nine in the first year—are mandatory. It’s important for patients to understand that these intravitreal injections improve the DRSS scores but do not have a major impact on visual acuity outcome.
 |
Laser vs. intravitreal therapy
The Early Treatment Diabetic Retinopathy Study6 results, first published in 1985, provided outcomes data on the role of focal- or grid-laser photocoagulation therapy on DME. In the pre-optical coherence tomography era, the ETDRS defined clinically significant macular edema (CSME) as retinal edema involving or threatening the center of the macula. The ETDRS showed 50 percent or greater reduction in the rates of moderate vision loss (MVL, equivalent to a doubling of the visual angle) in laser-treated eyes with CSME compared to untreated control eyes.
With the advent of OCT, our understanding of retinal pathology has improved tremendously. In the current OCT era, various clinical trials and DRCR Retina Network studies have utilized OCT to evaluate DME based on macular thickening of the central subfield. DME can be divided into two types: ci-DME and non-ci-DME.
Level I evidence supports focal/grid-laser treatment vs. no treatment for nci-DME. However, for ci-DME affecting visual acuity, a number of prospective clinical trials—VIVID/VISTA, RISE/RIDE, DRCR Retina Network Protocol T and RESTORE—have shown the beneficial role of intravitreal pharmacological agents to maintain or improve vision as well as anatomy on OCT. 7-10
DRCR Retina Network Protocol S
 |
This study evaluated the noninferiority of intravitreal ranibizumab (Lucentis, Roche/Genentech) compared with PRP in patients with PDR.11-14 This was a multicenter, randomized clinical trial conducted at 55 U.S. sites with 394 study eyes of 305 participants with PDR, VA 20/320 or better, and no history of PRP enrolled between February and December 2012 (mean age, 52 years; 44 percent female; 52 percent white).
Enrolled eyes were randomly assigned to receive PRP treatment, completed in one to three visits (n=203 eyes), or intravitreal ranibizumab 0.5 mg/0.05 ml at baseline and as frequently as q4 weeks based on a structured retreatment protocol (n=191 eyes). Eyes in both treatment groups could receive ranibizumab for DME. At two-year follow-up, mean VA letter improvement (primary outcome) was +0.2 in the PRP group vs +2.8 in the ranibizumab group.
It’s important to note that one eye in the ranibizumab group developed endophthalmitis. And at five years, VA outcomes were similar in the PRP and ranibizumab arms.
 |
DRCR Retina Network Protocol T
This study compared currently available anti-VEGF agents among DME eyes with baseline VA of 20/32 to 20/40.15 All three agents achieved a similar VA gain of approximately 1.5 lines at six months and maintained these gains through two years. For eyes with baseline VA 20/40 or better, visual outcomes were similar for all three anti-VEGF groups. However, among eyes with baseline VA of 20/50 or worse, while all three medications achieved robust VA gains—2 to 3 lines by month six and 24—aflibercept achieved both the greatest VA gain and largest central subfield thickness (CST) reduction.
Although aflibercept outperformed ranibizumab for visual outcomes at one year in eyes with worse baseline VA, no significant visual or anatomic differences between the two drugs were noted at year two. A post-hoc analysis of data of these patients showed that CST changes didn’t correlate well with VA changes.
DRCR Retina Network Protocol U
This Phase II randomized clinical trial compared treatment with ranibizumab alone and ranibizumab plus intravitreal dexamethasone implant (Ozurdex, Allergan) in 116 patients with persistent DME.16 Mean (standard deviation) improvement in visual acuity was 2.7 letters (9.8) in the combination group and 3 letters (7.1) letters in the ranibizumab group (p=0.73), while mean (SD) change in CST in the combination group was a loss of 110 μm (86) compared with a loss of 62 μm (97) for the ranibizumab-only group (p<0.001).
Nineteen eyes (29 percent) in the combination group experienced increased intraocular pressure or initiated treatment with antihypertensive eyedrops compared with none in the ranibizumab group (two-sided, p<0.001).
This study concluded that the addition of intravitreal dexamethasone to ranibizumab therapy doesn’t improve visual acuity at 24 weeks more than ranibizumab therapy alone among eyes with persistent DME following anti-VEGF therapy.
DRCR Retina Network Protocol V
Protocol V evaluated eyes with ci-DME and very good VA defined as a Snellen equivalent of 20/25 or better (electronic-ETDRS letter score >79).17 This study reported no significant difference in VA loss at two years, regardless whether eyes were initially managed with aflibercept, laser photocoagulation or observation.
The data disclosed that patients with ci-DME and very good visual acuity can be safely monitored for worsening of visual acuity before treatment.
Bottom line
We should consider all treatment options, keeping in mind the patient-specific factors, including anticipated visit compliance, cost and frequency of visits. In clinical practice, noncompliance among DR patients isn’t uncommon and may be caused by doctor visit fatigue secondary to multiple comorbidities, poor diabetes control, insurance issues, transportation difficulties and other factors. The treatment plan should be individualized and guided with the patient’s best interests in mind. RS
REFERENCES
1. Relhan N, Flynn HW Jr. The Early Treatment Diabetic Retinopathy Study historical review and relevance to today's management of diabetic macular edema. Curr Opin Ophthalmol. 2017;28:205-212.
2. Bakri SJ WJ, Regillo CD et al. Evidence-Based Guidelines for Management of Diabetic Macular Edema. J VitreoRetinal Dis. 2019;3:145-152.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf Updated February 14, 2020. Accessed March 27, 2020.
4. Solomon SD, Chew E, Duh EJ, et al. Erratum. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40:412-418.
5. Wykoff CC. PANORAMA: A Phase 3, double-masked, randomized study of efficacy and safety of aflibercept in patients with moderate to severe NPDR: Week 100 results. Paper presented at Angiogenesis, Exudation and Degeneration 2020; February 8, 2020; Miami, FL.
6. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796-1806.
7. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022.
8. Wykoff CC, Marcus DM, Midena E, et al. Intravitreal aflibercept injection in eyes with substantial vision loss after laser photocoagulation for diabetic macular edema: Subanalysis of the VISTA and VIVID randomized clinical trials. JAMA Ophthalmol. 2017;135:107-114.
9. Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376-2385.
10. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625.
11. Bressler SB, Beaulieu WT, Glassman AR, et al. Photocoagulation versus ranibizumab for proliferative diabetic retinopathy: Should baseline characteristics affect choice of treatment? Retina. 2019;39:1646-1654.
12. Hutton DW, Stein JD, Glassman AR, Bressler NM, Jampol LM, Sun JK, for the DRCR Retina Network. Five-Year Cost-effectiveness of intravitreous ranibizumab therapy vs panretinal photocoagulation for treating proliferative diabetic retinopathy: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2019:137:1424-1432.
13. Glassman AR, Beaulieu WT, Stockdale CR, et al. Effect of telephone calls from a centralized coordinating center on participant retention in a randomized clinical trial. Clin Trials. Published online January 27, 2020.
14. Maguire MG, Liu D, Glassman AR, et al., for the DRCR Retina Network. Visual field changes over 5 years in patients treated with panretinal photocoagulation or ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2020.
15. Bressler NM, Odia I, Maguire M, et al., for the DRCR Retina Network. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: A post hoc analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol. Published online June 27, 2019.
16. Maturi RK, Glassman AR, Liu D, et al., for the Diabetic Retinopathy Clinical Research Network. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: A DRCR Network Phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136:29-38.
17. Baker CW, Glassman AR, Beaulieu WT, et al., for the DRCR Retina Network. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA. 2019;321:1880-1894.



