 |
In reality, with the ever-increasing wave of aging and elderly patients in our nation, we have a burgeoning need to continue to work effectively together to manage our patients’ ophthalmic care. As our individual subspecialties advance at a fast pace, keeping up with important developments in other areas can be a daunting task. My goal here is to provide some brief but by no means exhaustive discussion of developments in cataract surgery.
 |
IOL technology
As a cataract surgeon, I am glad to be practicing in an era in which we have many options for treating our patients with the best possible intraocular lens technology, and these options continue to expand. Indeed, it can be difficult to sift through and discuss all the possibilities to arrive at best treatments for individual patients.
• Torics. The availability of toric IOLs potentially applies to millions of patients who have clinically significant astigmatism and require cataract removal. Toric IOL offerings allow the accurate correction of corneal astigmatism up to 4 to 4.5 D or even more when combined with bioptic approaches such as corneal relaxing incisions or subsequent LASIK. Clearly, the dramatic reduction in glasses dependence can be life altering for these patients.
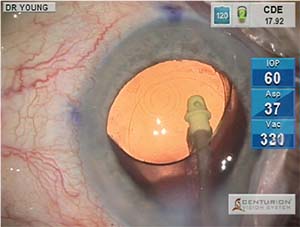 |
| Figure 1. A multifocal intraocular lens, the AcrySof ReStor +3.0 (Alcon), seen in red reflex microscope view following implantation in the capsular bag. |
It is gratifying for both surgeon and patient when a symptomatic cataract is cleared and the patient also has a newfound independence from glasses. Currently available toric IOLs show excellent long-term rotational stability when placed into the intact capsular bag.
Other than out-of-pocket expense for a “premium” IOL upgrade, few downsides argue against the use of toric implants, which unlike multifocal IOLs, are not associated with unwanted optical side effects or reduced contrast sensitivity. For this reason, toric IOLs can be used even in patients with significant comorbidities, like the many patients we share with our retinal colleagues. However, patients with severe limitations of visual potential following cataract removal may not find additional glasses independence worth the out-of-pocket cost.
• Multifocal and extended depth-of-focus IOLs. Very likely as a retinal specialist, you have found yourself peering through a condensing lens at a macula that was a little blurred by the multifocal IOL “in the way.” Maybe you have even thought something unkind about the cataract surgeon who put it there! Believe me, even though I am that guy on occasion, I think I speak for my colleagues when I say, “We feel your pain.”
The multifocal IOL can be an unfortunate circumstance when it finds its way into an eye with significant macular pathology or when a healthy macula subsequently develops pathology, as the small loss of contrast sensitivity and optical aberrations associated with multifocals may compound the vision loss due to the macular disease.
However, the multifocal IOL does play an important role in satisfying the vision needs of the cataract patient who desires spectacle independence. As cataract surgeons, we must use multifocal IOLs wisely—that is, in well-chosen candidates who are free of macular and ocular surface disease. Fortunately, we are seeing some continued evolution of the IOL technology that shows promise for improved vision results, greater flexibility for meeting the vision goals of our patients and reduced visual side effects.
One
 |
Regarding multifocal IOLs, we now have a wide array of choices from low-add to higher-add models (AcrySof ReStor, +2.5, +3.0, Alcon; and Tecnis Multifocal, ZKBOO, ZLBOO, Abbott) that allow targeting of individual patients’ specific vision goals (Figure 1). The lower-add IOLs tend to produce fewer problems with optical aberrations and less reduction of contrast sensitivity. A distance-dominant, low-add multifocal design (AcrySof ReStor +2.5 with ActiveFocus, Alcon) has been a welcome recent addition that produces very little degradation of distance clarity. The AcrySof ReStor +2.5 gives us a good alternative for our patients who have an active, outdoor lifestyle who want some near spectacle independence. It may be matched in some patients with a higher-add IOL in the nondominant eye for enhanced near function.
Last year the Food and Drug Administration approved the first multifocal toric IOL (AcrySof ReStor +3.0 Toric). It is available in four models to correct 0.75 to 2.82 D of corneal astigmatism. This development promises to be a great help in enhancing refractive precision in our multifocal patients, but also raises the bar in terms of the importance of accurate IOL placement and postoperative IOL stability.
Intraoperative Aberrometry
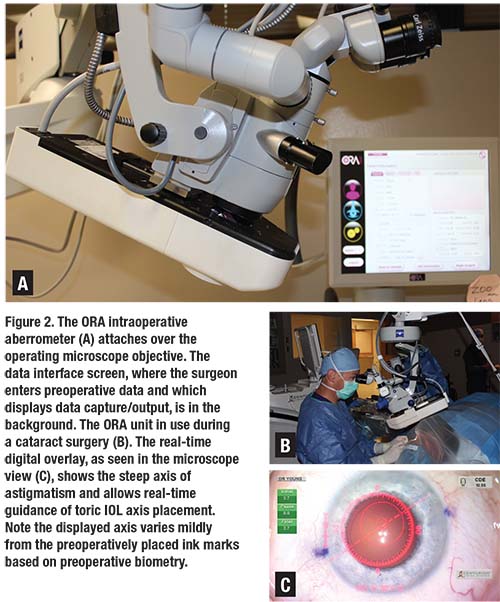
Accuracy of IOL selection and precision of toric IOL axis alignment play an increasing role in producing happy cataract surgery patients. Fortunately, we have some effective technologies that assist us in achieving these results. Intraoperative wavefront aberrometry has become an important tool in the operating room for many surgeons. In our facility, we use the ORA system (Alcon), the most commonly used system in the United States, which attaches to the operating microscope overlying the objective lens (Figure 2).
While careful preoperative biometry remains important, the ORA readings taken on the aphakic eye following cataract removal and prior to IOL implantation allow the further refinement of spherical IOL power and, importantly, give the surgeon a measurement of aphakic astigmatic power and axis. The real-time digital overlay displayed in the microscope oculars then assists the surgeon in achieving the correct IOL axis placement and takes post-implant readings to assure optimal IOL placement. Undoubtedly, intraoperative wavefront aberrometry technology will continue to improve and will play an increasing role in helping us provide excellent vision outcomes, especially as IOL technology continues to evolve and requires ever more precise biometry.
Femtosecond Laser-assisted Cataract Surgery
The arrival of femtosecond laser-assisted cataract surgery (FLACS) over the last five years as a viable option for routine cataract extraction continues to spark a debate about whether or not FLACS actually provides improved outcomes or added value over traditional manual cataract surgery. Clearly, femtosecond laser guidance by anterior segment optical coherence tomography in the current generation of lasers produces extremely precise corneal incisions for entry wounds or corneal relaxing incisions, perfectly circular and centered capsulorhexes, and pre-fragmented nuclei to ease subsequent phacoemulsification (Figure 3, page 20).
Without doubt, adoption of FLACS requires a large capital investment in equipment, additional costs for supplies and treatment royalty fees and additional time to complete a two-step surgery with a nonsterile laser component separate from a sterile intraocular cataract removal and IOL placement.
These additional costs require that patients pay more for FLACS to make the procedure feasible for surgeons and facilities. Usually, this cost is passed on to patients through premium IOL upgrade fees or enhanced cataract surgery refractive packages aimed at minimizing residual spherical and
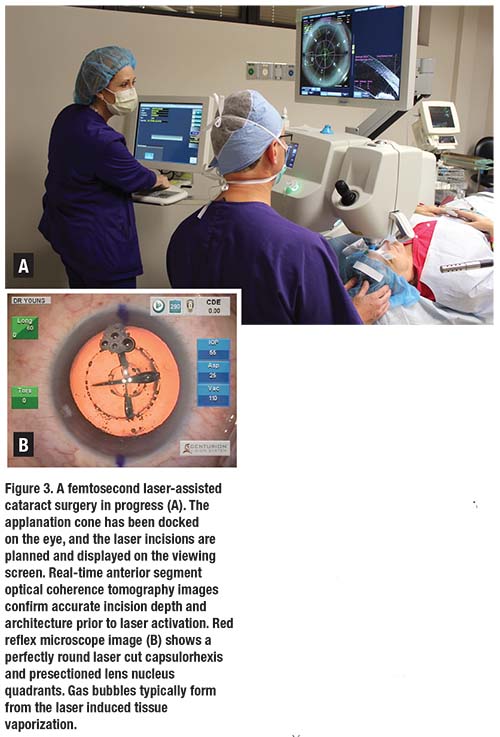 |
It was widely assumed at the outset of FLACS adoption that outcomes analysis would ultimately prove that the precision and perfection of laser cuts and probable reduction in necessary phacoemulsification energy translate into better visual outcomes and fewer surgical complications. However, current data are mixed at best and in many cases do not show clear outcomes advantage for FLACS over manual surgery.1 In some large series, intraoperative complication rates have been marginally higher in FLACS vs. manual surgery.2,3
The practical experience of many surgeons points to greater difficulty in certain surgical tasks in FLACS cases, particularly cortical removal. On the other hand, most surgeons agree that the precision of laser-created corneal relaxing incisions is a clear advantage over the manual technique.
Because of the unclear outcomes evidence, extra cost and time/patient- flow constraints, many surgeons—even those with appreciable FLACS experience—remain unconvinced of the added value of the technology and are at least selective about cases for which they use it.
Problematic Pupils
We cataract surgeons have all cringed at the appearance of the occasional cataract referral from our retinal colleagues. You know the one: diabetic, poorly dilating pupil, advanced cataract status post-vitrectomy, maybe a silicone oil fill (now or once present). (We really do appreciate these challenging opportunities!) I murmur to my assistant, “Please make this one a ‘last case’ of the day,” as I exit the exam lane.
The retinal surgeon and cataract surgeon do share some common frustrations with poorly dilating pupils that present difficulties with surgical visualization, as well as the unique challenges that may arise in eyes with considerable prior surgical history.
Indeed, with the ever more common use of alpha-1 adrenergic antagonists in clinical practice, added to the cases of pseudoexfoliation syndrome, diabetic denervation and uveitic synechiae formation, there are days when a surgeon wonders where have all the good pupils gone. Fortunately, we do have some useful tools to employ today that make these tougher cases seem a little easier and more routine.
One very useful recent innovation is the use of intracameral phenylephrine 1% and ketorolac 0.3% (Omidria, Omeros Corporation). Omidria is available as an addition to the infusion fluid during cataract surgery and is FDA-approved for the maintenance of intraoperative pupillary dilation and reduction of postoperative pain. In my own experience, it noticeably improves dilation and maintenance of dilation in cases that in the past would have been more difficult due to loss of an adequate pupil (Figure 4A). A recent study by Eric Donnenfeld, MD, demonstrated decreased surgical time, reduced intraoperative complications, reduced use of pupil-expansion devices and improved postoperative best-corrected visual acuity compared to the use of intracameral epinephrine alone.4 (Dr. Donnenfeld is a consultant to Omeros.)
Certainly, cases still arise where severe floppy iris syndrome or significant posterior synechiae make additional pupil expansion necessary. Convenient preloaded, injectable pupil expansion rings are my go-to solution in these cases. These self-retaining rings (Malyugin rings, available commercially from several manufacturers) may be rapidly placed to maintain a fixed pupil opening during surgery and then rapidly and atraumatically removed following cataract removal and IOL placement (Figure 4B). In my own practice, I am using many fewer rings due to increased use of Omidria, in agreement with Dr. Donnenfeld’s study.
Infection Prophylaxis
Every cataract surgeon dreads that call from the five-day postoperative cataract patient who was 20/20 and smiling at day one postoperatively and now reports a red, painful eye with very poor vision.
All of our best efforts at state-of-the-art IOL technology, precise and accurate biometry and skilled surgery can be undone by endophthalmitis. This concern has led many surgeons to consider infection prophylaxis measures that go beyond the traditional Betadine lid prep with topical antibiotic drops given peri- and postoperatively. Increasingly, cataract surgeons are adopting the use of intracameral antibiotic dosing at the completion of a case.
Intracameral Antibiotics
There is convincing evidence from multiple large case series that instituting intracameral antibiotics during cataract surgery markedly reduces the incidence of endophthalmitis.5,6 A fivefold to tenfold reduction of endophthalmitis risk has been reported.
A variety of intracameral antibiotics have been used, most commonly cefuroxime, moxifloxacin and vancomycin. Indeed, intracameral antibiotic prophylaxis is considered standard of care throughout Europe, where a commercial preparation of cefuroxime for intracameral injection is available (Aprokam, Laboratories Thea).
Increasingly, U.S. surgeons are also adopting
 |
| Figure 4. A typical atrophic iris appearance induced by use of tamsulosin (Flomax, Boehringer Ingelheim) (A). In this case, intraoperative use of phenylephrine 1%/ketorolac 0.3% (Omidria, Omeros) in the balanced salt solution infusion helps maintain acceptable pupillary dilation. In the management of floppy iris syndrome (B), a Malyugin ring helps maintain adequate pupillary aperture. |
The intracameral use of commercially available moxifloxacin (Vigamox, Alcon), which is nonpreserved in the bottle and is withdrawn either undiluted or diluted in balanced salt solution, has been well described for over a decade, has been used widely and is without reported problems.7 Another problem is that current Medicare/third-party regulations require surgeons or facilities to absorb the cost of the antibiotic given as part of a bundled reimbursement, which further increases per-case costs in an era of declining reimbursement.
Intravitreal Medications
The frequent difficulties that many of our predominantly elderly surgery patients have with their postoperative eye drop regimens—multiple bottles, multiple and often variable dose frequency, not to mention ever-growing expense—has led many to consider postoperative strategies to reduce or eliminate these regimens.
One common strategy for so-called “dropless” cataract surgery involves the intravitreal injection of 0.2 ml of a compounded formulation of triamcinolone acetonide (15 mg/ml), moxifloxacin (1 mg/ml) and vancomycin (10 mg/ml) (Imprimis Pharmaceuticals) by either pars plana injection or a transzonular infusion using a 30-gauge cannula. In transzonular infusion, a bent 30-gauge cannula is used to traverse the anterior chamber via the cataract incision, then penetrate the zonules via the ciliary sulcus to infuse the drug mixture into the anterior vitreous.8
However, anecdotal reports of persistent visual haze and floaters noticeable to patients and retinal complications, including retinal detachments, may deter wider adoption. Alternatively, other “dropless” regimens may simply employ an intracameral antibiotic dose with an injection of sub-Tenon or subconjunctival triamcinolone, avoiding the potential for floaters or vitreoretinal complications. Concern for intraocular pressure rise that may be difficult to control in some patients is a significant negative with these regimens. RS
REFERENCES
1. Kent, C. Update: Is FLACS better than manual surgery? Rev Ophthalmol. 2017;24(3) 22-31.
2. Manning S, Barry P, Henry Y, et al. Femtosecond laser-assisted cataract surgery vs. standard phacoemulsification cataract surgery: Study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2016;42:1779-1790.
3. Abell RG, Darian-Smith E, Kan JB, Allen PL, Ewe SYP, Vote JB. Femtosecond laser-assisted cataract surgery vs. standard phacoemulsification cataract surgery: Outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015; 41: 47-52
4. Charters L, Donnenfeld ED. Intracameral phenylephrine/ketorolac superior to intracameral epinephrine. Ophthalmol Times. 2017:42(2);14-15
5. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multi-center study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
6. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013; 39:8-14.
7. Braga-Mele R, Chang DF, Henderson BA, et al., for ASCRS Clinical Cataract Committee. Intracameral antibiotics: Safety, efficacy, and preparation. J Cataract Refract Surg 2014;40:2134-2142.
8. Tyson SL, Bailey R, Roman JS, Hark LA, Haller JA. Clinical outcomes after injection of a compounded pharmaceutical for prophylaxis after cataract surgery: a large-scale review. Curr Opin Ophthalmol. 2017;28:73-80.



