| ABOUT THE AUTHORS | |
 | Dr. Toussaint is a retina fellow at Cincinnati Eye Institute and University of Cincinnati Department of Ophthalmology. |
 | Dr. Shaikh is a vitreoretinal surgeon at Houston Eye Associates in Texas. |
 | Dr. Sisk performs adult and pediatric vitreoretinal surgery at Cincinnati Eye Institute and is an associate professor of ophthalmology at the University of Cincinnati. DISCLOSURES: Dr. Sisk is a speaker for Heidelberg Engineering. Drs. Toussaint and Shaikh have no disclosures. |
Pathophysiology of CSCR
CSCR is a common form of exudative retinopathy typically affecting men between ages 30 and 50 years. It involves focal or multifocal leaks at the level of the retinal pigment epithelium (RPE), usually manifesting as subretinal fluid and pigment epithelial detachment. We don’t fully understand the pathogenesis of CSCR, but it appears to be related to hyperpermeability at the RPE associated with vascular congestion and thickening of the choroidal circulation.1Symptomatic patients experience micropsia, metamorphopsia and reduced visual acuity. The vast majority of patients experience a single acute episode that spontaneously resolves after three to six months with improvement to baseline visual acuity.1 However, one-third of patients develop chronic or recurrent disease that may create persistent vision impairment from damage to the RPE and photoreceptors.
Non-idiopathic Disease
Most cases of CSCR have been classified as idiopathic. However, steroids have been demonstrated to cause or perpetuate CSCR, particularly in patients who have elevated serum cortisol levels such as those in Cushing’s syndrome, pregnancy or exogenous steroid treatments.2 Reports have also described associations with stress and type A personality, which may be associated with elevated blood cortisol. Case reports have also documented associations with Helicobacter pylori, phosophodiesterase-5 inhibitors and obstructive sleep apnea.Once the diagnosis of CSCR is made, the most common intervention is to recommend lifestyle changes.
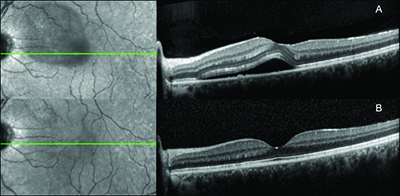
who had consumed eight cups of coffee per day (A) and a follow-up OCT
image (B) three months after the patient stopped consuming caffeine. This
patient had no clinical recurrence of disease.
Additional treatments for CSCR include photodynamic therapy (PDT), argon laser, micropulse laser,3 rifampin (Rifadin),4 the antimineralocorticoids spironolactone (Aldactone)5,6 and eplerenone (Inspra), and anti-glucocorticosteroid therapy (mifepristone, ketoconazole and finasteride). Evidence supporting these therapies has mostly been in small, uncontrolled, retrospective case series. One exception is argon laser photocoagulation.7 While short-term results seemed promising, significant recurrence rates8 and the visual sequelae of macular laser have left ophthalmologists searching for alternative therapies.
Role for Guided PDT
| The Mechanism of Caffeine in CSCR |
What is the mechanism by which caffeine can produce central serous chorioretinopathy (CSCR)? Surprisingly, this is a function of a link between caffeine and endogenous corticosteroids. Patients with CSCR have statistically higher endogenous cortisol levels than age-matched controls.19  Caffeine consumption elevates both ACTH and cortisol concentrations relative to placebo.21 One proposed mechanism is that caffeine blocks adenosine receptors. Adenosine (A1) receptors accelerate the removal of subretinal fluid while A2 receptors slow this removal.22 Caffeine is a potent antagonist of adenosine. Chronic caffeine consumption (600 mg/day for one week or 400 mg/day for two weeks) upregulates A2 receptors.23 Interestingly, subjects treated with 400 mg/day of caffeine for one week did not experience changes in adenosine receptor activation. It is quite possible that this dose-dependent effect of caffeine consumption might also result in a dose-dependent change in choroidal thickness and in the balance of A1:A2 receptors contributing to fluid movement across the RPE. Although most Americans consume some caffeine daily,17,18 it remains unclear why only some of those who abuse caffeine develop CSCR. Likely genetic factors predispose certain individuals. While a genetic link between caffeine and CSCR has yet to be solidified, genetic variants in complement factor H24 as well as in the age-related macular susceptibility (ARMS2) gene have shown an association with chronic CSCR.25 Risk alleles for CSCR may be protective in age-related macular degeneration.25 |
Most patients with CSCR do not have classic risk factors. We have observed significant caffeine consumption among many of our acute and chronic “idiopathic” CSCR patients. Caffeine use is ubiquitous: 80 percent of Americans are reported to ingest caffeine in some form daily.17
Experts vary regarding how much caffeine is safe, ranging between 200 and 400 mg/day (approximately two to four 8-oz. cups of regular coffee).17,18 The Food and Drug Administration and other leading health authorities state that caffeine abuse—consumption of greater than approximately 500-600 mg of caffeine daily (equivalent to four to seven cups of coffee)—is associated with cardiac dysrhythmia, psychological alterations, dehydration and, in extreme cases, death.17,18
Manifestations of Caffeine Abuse
We investigated the association between CSCR and caffeine with an Institutional Review Board-approved retrospective case series. We reviewed the records of 76 patients diagnosed with CSCR between 2010 and 2013 at the Cincinnati Eye Institute by a single provider. Twenty-one (28 percent) patients reported exposure to any type of steroid, although we did not think all exposures were causative. Of the 55 cases not associated with steroid use, 38 (69 percent) of patients noted consumption of caffeine. For 20 (36 percent) of the 55 patients, caffeine was the only risk factor for development of CSCR.Thirty-eight of the caffeine consumers with non-steroid induced CSCR self-reported “moderate” or greater consumption of caffeine or four cups of coffee per day or more; we found that 24 (63 percent) of them abused caffeine. Among the caffeine abusers, seven (29 percent) had bilateral disease, 13 (54 percent) exhibited subretinal fluid, and five (21 percent) had a pigment epithelial detachment. We noted bilateral RPE changes in eight of the 24 (33 percent) patients and unilateral RPE changes in 15 (63 percent).
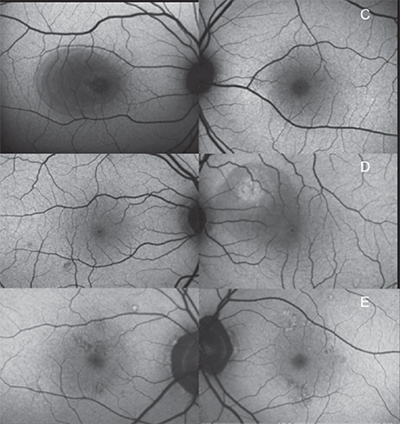
caffeine with bilateral central serous chorioretinopathy and high caffeine
intake: a 48-year-old male with a serous retinal detachment who consumed
a half-gallon of Mountain Dew per day (top); 30-year-old male with subretinal
fluid who drank six cans of Mountain Dew daily as well as supplemental
energy drinks (center); and a 53-year-old male with bilateral RPE changes who
consumed four to five cups of coffee per day (bottom).
Our series suffered from lack of follow-up, as five patients were lost to follow-up after their first visit and eight patients were lost after their three-month visit.
Nine patients overall, five of whom had more than three months of follow up, underwent reduced-fluence PDT. One patient did not have resolution of subretinal fluid despite PDT until after cessation of caffeine.
Figure 1 shows a baseline OCT image of one patient with subfoveal fluid who was consuming eight cups of coffee per day. (Figure 1A) A follow-up OCT image (Figure 1B) three months after the patient stopped consuming caffeine shows reduction of subretinal fluid. This patient had no clinical recurrence of disease.
Figure 2 shows fundus autofluoresence images of the macula of both eyes of patients with bilateral CSCR and high caffeine intake. The patient in Figure 2 top is a 48-year-old male who consumed a half gallon of Mountain Dew per day. He had central macular hypoautofluorescence from a serous retinal detachment in the right eye.
Figure 2 center shows a 30-year-old male who drank six cans of Mountain Dew per day as well as supplemental energy drinks. The right eye has small focal areas of macular hypoautofluorescence, and the left eye had hyperautofluorescence associated with serous retinal detachment
The patient in Figure 2 bottom is a 53-year-old male with bilateral irregular hyper- and hypoautofluorescent RPE changes who consumed four to five cups of coffee per day.
| Take-home Point |
| Central serous chorioretinopathy (CSCR) has well-established risk factors for its presentation. However, these are infrequently present. In our patient population, many who presented CSCR also abused caffeine. We propose that chronic, excessive caffeine intake may be a modifiable risk factor in patients diagnosed with CSCR and may be responsible for many “idiopathic” cases. Future studies on CSCR should evaluate the role of caffeine abuse. |
References
1. Colucciello M. Update on central serous chorioretinopathy. Retin Phys. 2012;9:42-46.
2. Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: A pathogenetic model. Clin Ophthalmol. 2011:5 239-243.
3. Kim JY, Park HS, Kim SY. Short-term efficacy of subthreashold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015 Feb 26 [epub ahead of print].
4. Shulman S, Goldenberg D, Schwartz R, et al. Oral rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015 Mar 21 [epub ahead of print].
5. Ryan EH, Pulido CM. Treatment with spironolactone: A challenge-rechallange case. Retin Case Brief Rep. 2015;9:235-2398.
6. Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretiopathy—a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252:1985-1991.
7. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979; 63:674-677.
8. Ficker L, Afidis G, While A, Leaver P. Long-term follow up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72:829-834.
9. Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: One-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756-1765.
10. Ruiz-Moreno JM, Lugo FL, Armada F, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol. 2010;88:371-376.
11. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv Ophthalmol. 2013; 58:103-126.
12. Reibaldi M, Cardascia N, Long A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010; 149:307-315.
13. Tseng CC, Chen SN. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol. 2015 Feb 13 [epub ahead of print].
14. Kim YK, et al. Comparison of visual and anatomical outcomes of half-fluence and half-dose photodynamic therapy in eyes with chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;160:72-84.
15. Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014;92:e594-601.
16. Alkin Z, Perente I, Ozkaya A, et al. Comparison of efficacy between low-fluence and half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Clin Ophthalmol. 2014;8:685-690.
17. FDA resources page. Food and Drug Administration Web Site. Understanding over-the-couner medicines. Available at: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/. Accessed September 1, 2015.
18. Mayo Clinic resources page. Nutrition and healthy eating: Caffeine: How much is too much? Available at: http://www.mayoclinic.org/healthy-living/nutrition-and-healthy-eating/in-depth/caffeine/art-20045678. Accessed August 12, 2015.
19. Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962-964.
20. Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary adrenal axis in adult retinal pigment epithelium. J Endocrinol. 2007;193:157-169.
21. al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998; 60:521-527.
22. Wan WJ, Cui DM, Yang Z, et al. Expression of adenosine receptors in human retinal pigment epithelium cells in vitro. Chin Med J. 2011;124:1139-1144.
23. Varani K, Portaluppi F, Gessi S, et al. Dose and time effects of caffeine intake on human platelet adenosine A2a receptors. Circulation. 2000;102: 285-289.
24. Miki A, Kondo N, Yanagisawa S Bessho H Honda S, Negi A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121:1067-1072.
25. de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122:562-570.
2. Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Central serous chorioretinopathy: A pathogenetic model. Clin Ophthalmol. 2011:5 239-243.
3. Kim JY, Park HS, Kim SY. Short-term efficacy of subthreashold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015 Feb 26 [epub ahead of print].
4. Shulman S, Goldenberg D, Schwartz R, et al. Oral rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015 Mar 21 [epub ahead of print].
5. Ryan EH, Pulido CM. Treatment with spironolactone: A challenge-rechallange case. Retin Case Brief Rep. 2015;9:235-2398.
6. Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretiopathy—a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252:1985-1991.
7. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979; 63:674-677.
8. Ficker L, Afidis G, While A, Leaver P. Long-term follow up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72:829-834.
9. Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: One-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756-1765.
10. Ruiz-Moreno JM, Lugo FL, Armada F, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol. 2010;88:371-376.
11. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv Ophthalmol. 2013; 58:103-126.
12. Reibaldi M, Cardascia N, Long A, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010; 149:307-315.
13. Tseng CC, Chen SN. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol. 2015 Feb 13 [epub ahead of print].
14. Kim YK, et al. Comparison of visual and anatomical outcomes of half-fluence and half-dose photodynamic therapy in eyes with chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;160:72-84.
15. Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014;92:e594-601.
16. Alkin Z, Perente I, Ozkaya A, et al. Comparison of efficacy between low-fluence and half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Clin Ophthalmol. 2014;8:685-690.
17. FDA resources page. Food and Drug Administration Web Site. Understanding over-the-couner medicines. Available at: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/. Accessed September 1, 2015.
18. Mayo Clinic resources page. Nutrition and healthy eating: Caffeine: How much is too much? Available at: http://www.mayoclinic.org/healthy-living/nutrition-and-healthy-eating/in-depth/caffeine/art-20045678. Accessed August 12, 2015.
19. Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962-964.
20. Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary adrenal axis in adult retinal pigment epithelium. J Endocrinol. 2007;193:157-169.
21. al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998; 60:521-527.
22. Wan WJ, Cui DM, Yang Z, et al. Expression of adenosine receptors in human retinal pigment epithelium cells in vitro. Chin Med J. 2011;124:1139-1144.
23. Varani K, Portaluppi F, Gessi S, et al. Dose and time effects of caffeine intake on human platelet adenosine A2a receptors. Circulation. 2000;102: 285-289.
24. Miki A, Kondo N, Yanagisawa S Bessho H Honda S, Negi A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121:1067-1072.
25. de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122:562-570.



