Take-home points
|
 |
|
Bios Dr. Shaheen is with the Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami. Dr. Danzig is with the Rand Eye Institute, Deerfield Beach, Florida, and Florida Atlantic University, Charles E. Schmidt School of Medicine, Boca Raton. DISCLOSURES: Dr. Shaheen has no relevant disclosures. Dr. Danzig disclosed relationships with Adverum Biotechnologies, Alexion, Bayer, Genentech/Roche, Gyroscope Therapeutics, IvericBio, Kodiak Sciences, Novartis, Regeneron Pharmaceuticals and Unity Biotechnology. |
Retinal vein occlusion is the second most common cause of retinal vascular diseases after diabetic retinopathy.1 It’s defined by the site of occlusion as branch RVO, when the occlusion occurs at a venous tributary; hemiretinal vein occlusion, when it occurs at the major bifurcation of the retinal vein; and central RVO, when it occurs within or posterior to the optic nerve head.1 The etiology of RVO includes impaired venous return and its manifestation ranges from asymptomatic to complete visual loss.1
BRVO is subdivided into major and macular BRVO. Major BRVO presents with a peripheral field defect due to an occlusion of a retinal vein. Macular BRVO presents with central field defect due to occlusion of a macular vein. In both subtypes, visual acuity can improve without treatment by three or more lines, yet the two subtypes have different final visual outcomes.2
In contrast, VA in eyes with CRVO may range from excellent vision in the absence of retinal nonperfusion to severe vision loss if the retinal nonperfusion is substantial.3 Eyes with mild-to-moderate nonperfusion may go on to develop progressive nonperfusion with potential further complications, making their future visual outcomes more challenging to predict.3
Moving from surgery to anti-VEGF
Several interventions aimed at enhancing perfusion and relieving venous blockage have proven ineffective. These include creating anastomoses by surgery and laser, thrombolysing the occlusion and bypassing the congestion via optic nerve sheathotomy. For this reason, the management revolves around treating and preventing RVO complications.
RVO can cause macular edema and/or ischemia, vitreous hemorrhage, tractional retinal detachment and neovascular glaucoma. Clinical trials for RVO initially focused on laser photocoagulation, which demonstrated effectiveness in BRVO but limited efficacy for CRVO. In 2009 the dexamethasone steroid injection (Ozurdex, Allergan/AbbVie) became the first intravitreal medication to be specifically approved by the Food and Drug Administration to treat macular edema from RVO.
Bevacizumab (Avastin, Genentech/ Roche) was used off-label for RVO as early as 2005.4 FDA approval of ranibizumab (Lucentis, Genentech/Roche) and aflibercept (Eylea, Regeneron Pharmaceuticals) for RVO followed Ozurdex a few years later. More recently, two Phase III studies of faricimab (Vabysmo, Genentech/Roche) have demonstrated equivalency to aflibercept in treating BRVO and CRVO, and intravitreal tarcocimab (KSI-301, Kodiak Sciences) is being evaluated for macular edema due to RVO. Here, we explore the evidence supporting the FDA-approved treatments for RVO.
RVO in Clinical Practice Though retinal vein occlusion is often a straightforward diagnosis, treatment can remain burdensome, leaving some patients significantly undertreated. Treatment algorithms vary amongst retina specialists, and often they don’t exactly align with the clinical trial paradigms. Many factors enter into the treating physician’s mind. What’s the presenting vision? Is the RVO ischemic or nonischemic? Is there an afferent pupillary defect? Is the patient phakic? Can I appreciate any neovascularization, particularly of the iris? Does the patient need prior authorization in order to initiate therapy? All of these factors play a role in how we treat our patients. Off-label bevacizumab (Avastin, Genentech/Roche) may be the first treatment, or occasionally the only allowed treatment, for a patient with macular edema due to RVO. That may not be ideal for many patients, but it’s absolutely a reasonable start. Furthermore, all anti-VEGF injections can suppress the downstream consequences of RVO such as possible vitreous hemorrhage, neovascular glaucoma and even traction retinal detachment that would possibly occur had the disease been left untreated. Both ranibizumab (Lucentis, Genentech/Roche) and aflibercept (Eylea, Regeneron Pharmaceuticals) can maintain good control of macular edema in many patients, but some need more. These patients often benefit from adding a dexamethasone implant (Ozurdex, Allergan/AbbVie) to their regimen. Yet, still some patients need focal laser or panretinal photocoagulation depending on the level of ischemia and nonperfusion seen on fluorescein angiography. Role of imaging When I first encounter a new patient with any type of RVO, I sometimes get a widefield fluorescein angiogram if I feel the quality will be adequate to identify areas of ischemia and the information would be useful, but more often than not I defer FA until I have better visualization of the retina tissue once the degree of hemorrhage lessens. All I need most of the time is a quality, comprehensive examination and an optical coherence tomography scan. From there, in most cases I can initiate therapy on the same day. Many of these patients have commercial insurance and all too often I’m forced to use off-label bevacizumab as my initial therapy. If I had my choice, and if it were available, I would use a sample of aflibercept as my first-line treatment, and I would continue it monthly for at least three doses. Often, I continue monthly for up to six doses, though I may use a treat-and-extend protocol following the third or fourth injection, depending on how the patient is doing. During this time, between months three and six, I commonly order a widefield FA to assess peripheral nonperfusion. Adding dexamethasone implant If a patient can't go longer than six to eight weeks between anti-VEGF, namely aflibercept, injections, I’ll usually add a dexamethasone implant. A steroid injection isn’t a replacement for anti-VEGF therapy, but rather a supplement to it. Steroid treatments come with their share of side effects. More than one intravitreal steroid treatment will cause cataract progression in a phakic patient. Intraocular pressure rise is also of concern, but it only occurs in a subset of patients. Laser isn’t commonly used, although it may decrease the vascular endothelial growth factor load in a patient with significant peripheral ischemia, as identified on widefield FA. Overall, most patients do quite well, and we’re no longer seeing nearly as much neovascular complications that we did before the anti-VEGF era. Nevertheless, more often than we likely realize, patients are undertreated in the real world, with the current burden of injections too difficult for some patients to manage. —C.D. |
Ranibizumab in BRVO
 |
The BRAVO5 and CRUISE6 trials, respectively, evaluated ranibizumab for efficacy and safety for BRVO- and CRVO-associated macular edema. BRAVO enrolled 397 patients receiving a 1:1:1 pattern of monthly intraocular injections of 0.3 or 0.5 mg ranibizumab or sham. The mean (95% CI) change in Early Treatment Diabetic Retinopathy Study letter score in BCVA at six months was 16.6 (14.7 to 18.5) and 18.3 (16.0 to 20.6) in the 0.3- and 0.5-mg treatment groups, and 7.3 (5.1-9.5) in the sham group (p<0.0001 for each ranibizumab group vs. sham). The proportion of patients who gained ≥15 letters at month six was 55.2 and 61.1 percent in the respective ranibizumab groups, and 28.8 percent in the sham arm (p<0.0001). At month six, significantly more ranibizumab-treated patients (67.9 and 64.9, respectively) had BCVA of ≥20/40 than sham patients (41.7 percent; p<0.0001). Central foveal thickness (CFT) decreased by a mean of 337 and 345 µm in the ranibizumab groups and 158 µm in the sham group (p<0.0001).
Ranibizumab in CRVO
The CRUISE trial included 392 CRVO patients with macular edema and the design was similar to that of BRAVO. The mean (95% CI) change from baseline BCVA letter score at month six was 12.7 (9.9 to 15.4) and 14.9 (12.6 to 17.2) in the 0.3 mg and 0.5 mg ranibizumab groups, respectively, and 0.8 (-2 to 3.6) in the sham group (p<0.0001).
The percentage of patients who gained ≥15 letters in BCVA at month six was 46.2 and 47.7 percent in the respective ranibizumab groups and 16.9 percent in the sham group (p<0.0001). At six months, CFT decreased by a mean of 434 µm (0.3 mg) and 452 µm (0.5 mg) vs. 168 µm in the sham group (p<0.0001). The FDA approved ranibizumab 0.5 mg for treating macular edema following RVO in 2010.
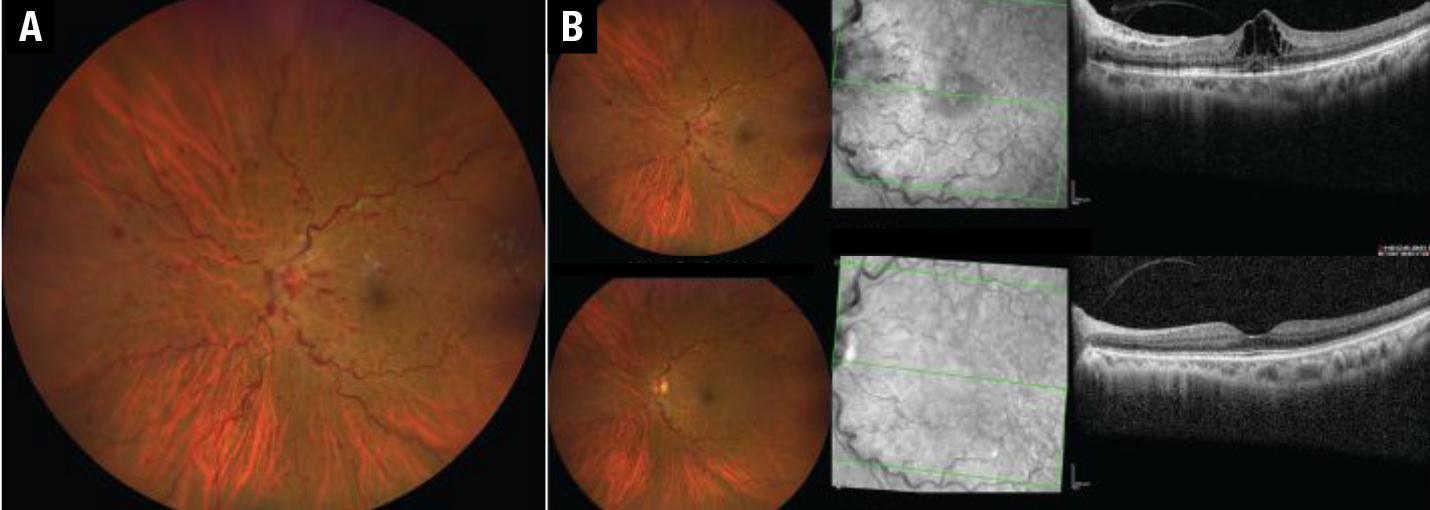 |
| Figure 1. A) Color fundus photograph and corresponding optical coherence tomography scan of an eye with central retinal vein occlusion before aflibercept injections and B) six months after treatment. Notice resolution of the hemorrhages, optic disc swelling and the decrease in vessel tortuosity and dilatation. |
Aflibercept for RVO
The VIBRANT trial evaluated aflibercept for efficacy and safety against macular grid laser for BRVO-associated macular edema (Figure 1).7 In the aflibercept group, 52.7 percent gained ≥ 15 ETDRS letters at the week 24 vs. 26.7 percent in the laser group (p=0.0003), with a mean improvement of 17 ETDRS letters in the former and 6.9 ETDRS letters in the latter (p<0.0001).
Aflibercept was also evaluated for CRVO-associated macular edema in the COPERNICUS trial involving 188 patients.8 Trial subjects received aflibercept injection 2 mg or sham injections every four weeks up to week 24. During weeks 24 to 52, patients in both arms received aflibercept injection pro re nata with monthly follow-up. In weeks 52 to 100, subjects also received aflibercept injections PRN but with at least every three months follow-up evaluation. At week 25, 56.1 vs. 12.3 percent gained ≥ 15 ETDRS letters; at week 52, the respective gains were 55.3 vs. 30.1 percent; and at week 100, 49.1 vs. 23.3 percent (p<0.001 for all).
The mean change from baseline BCVA was also significantly higher in the treatment arm: +17.3 vs. –4 letters at week 24 (p<0.001); +16.2 vs. +3.8 at week 52 (p<0.001); and +13 vs. +1.5 at week 100 (p<0.0001). At the same time points, the mean central retinal thickness reduction from baseline was 457.2 vs. 144.8 μm (p<0.001); 413 vs. 381.8 μm (p=0.546); and 390 vs. 343.3 μm (p=0.366) in the treatment vs. the control arms, respectively. The FDA in 2014 approved aflibercept for treating BRVO or CRVO with macular edema.
Clinical trials vs. the real world
As these results show,8-11 frequent anti-VEGF injections for macular edema associated with BRVO or CRVO have improved the mean VA in randomized clinical trials. While RCTs are the most reliable research design for evaluating the effectiveness of interventions, what matters is how this translates into real-world practice. Real-world treatment involves variable treatment patterns, patient groups, care providers and, more importantly, follow-up intervals.
Real-world studies on the approved anti-VEGF treatments for RVO-associated macular edema have demonstrated outcomes that diverge from the clinical trials. We evaluated BCVA outcomes and anti-VEGF injection frequency during the first year of treatment and improvement of macular edema in eyes with BRVO or CRVO in real-world studies vs. randomized clinical trials.
We also incorporated the published results of long-term extension (LTE) studies.12 We included seven controlled clinical trials (two BRVO, five CRVO; all with monthly monitoring), four LTE trials (two BRVO/CRVO, two CRVO; the majority with less than monthly monitoring); and two real-world studies (both BRVO/CRVO) monitored at the investigator’s discretion.
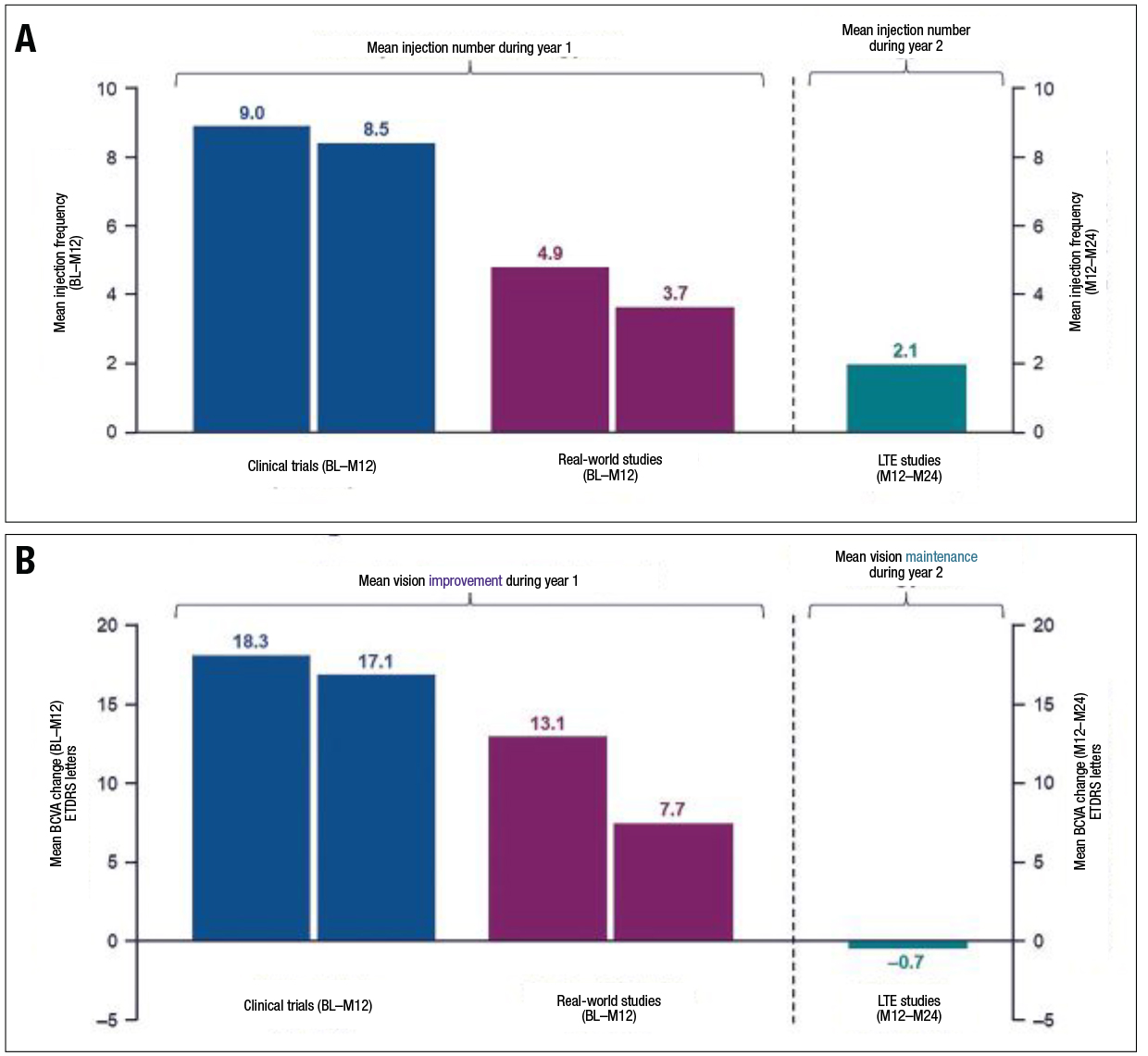 |
| Figure 2. A) Patients with branch retinal vein occlusion in long-term extension (LTE) and real-world studies received fewer injections on average over 12 months than those in clinical trials. B) In addition, compared to clinical trials, patients with BRVO in real-world studies had a lesser improvement in visual acuity. In contrast, patients retained the initial vision improvements gained in clinical trials during LTE studies. (Courtesy Genentech/Roche) |
Lower injection frequency
We found that the anti-VEGF injection frequency in patients with branch RVO was lower in real-world studies13,14 and LTE trials15 than in clinical trials (Figure 2A).9,16,17 In real-world studies, individuals with BRVO who had fewer anti-VEGF injections didn’t attain the visual benefits found in clinical trials. In contrast, individuals who participated in LTE studies were able to sustain their initial vision gains made during clinical trials (Figure 3B).
Similar to what we observed in patients with BRVO, patients with CRVO received, on average, fewer anti-VEGF injections throughout the course of 12 months in real-world studies13,14 and LTE trials8,15,18 than in clinical trials (Figure 3A).8,10,11,17-20 In contrast to patients with BRVO, patients with CRVO were unable to preserve their initial visual improvements throughout long-term extension studies (Figure 3B).
Therefore, there’s now an unmet need for longer-acting drugs that can address the varying intervals between visits and injections that we encounter in the real world.
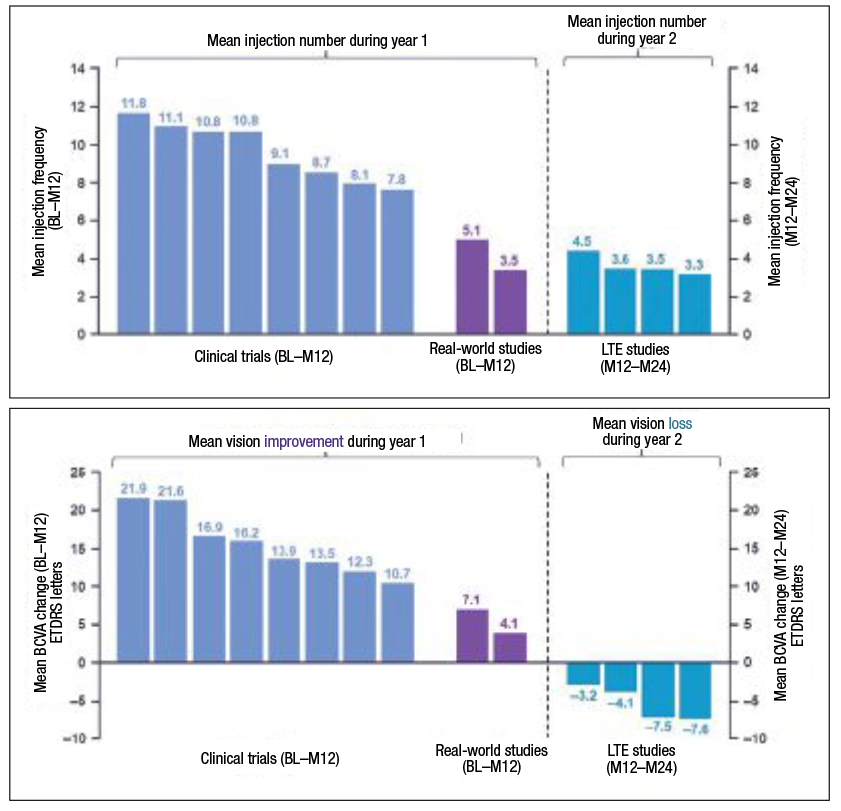 |
| Figure 3. A) Patients with central retinal vein occlusion who participated in long-term extension (LTE) and real-world evidence studies got fewer injections over 12 months than those who participated in clinical trials. B) In addition, in real-world studies, patients with CRVO achieved lower vision improvements than in clinical trials. Still, in LTE studies, patients didn’t retain the initial vision gains seen in clinical trials. (Courtesy Genentech/Roche) |
Faricimab emerges
Faricimab, a bispecific antibody with a novel mechanism of action that targets angiopoietin-2 and VEGF-A, is being evaluated against aflibercept for macular edema associated with BRVO in the BALATON21 and CRVO in the COMINO trials.22 Both studies have two treatment arms:
- faricimab 6 mg given at fixed four-week intervals for 20 weeks (part 1), followed by a personalized treatment interval (PTI) dosing regimen from week 24 to week 72 (part 2); and
- aflibercept 2 mg at four-week intervals through week 20 (part 1), followed by faricimab 6 mg PTI from week 24 through week 72 (part 2).
The primary endpoint for these studies is the average change in BCVA score from baseline through week 24.
Genentech/Roche recently reported topline results that showed both studies met their primary endpoint of noninferior VA gains compared to aflibercept. The trial also reported that faricimab showed rapid drying of retinal fluid, as measured by reduction in central subfield thickness, as well as a safety profile in line with previous trials. Both studies are expected to end in June 2023.
Tarcocimab
Another novel drug, intravitreal tarcocimab (KSI-301, Kodiak Sciences), is being evaluated for macular edema due to RVO in the BEACON study,23 estimated to conclude in July 2023. It has 568 participants randomized 1:1 to KSI-301 or aflibercept. Initial results showed that tarcocimab is the first anti-VEGF therapy to show non-inferior VA outcomes with fewer injections than used, on average, in clinical practice.24
Bottom line
Patients with macular edema from BRVO and CRVO had considerable BCVA improvements in RCTs during the first year with frequent anti-VEGF injections and frequent monitoring.
In long-term extension studies (months 12 to 24), however, patients with BRVO retained the initial visual gains attained during the clinical trials, but those with CRVO couldn’t retain their vision improvements, and their mean BCVA decreased. Patients in real-world studies didn’t achieve the same BCVA improvements reported in clinical trials over the first year.
These findings highlight the need for treatments with longer therapeutic action to lessen the treatment burden and the necessity for frequent monitoring and, more specifically, to bring the real-world practice results closer to randomized control trials. There’s a recent renewed interest in this endeavor, as evidenced by the ongoing Phase III trials of faricimab, which has already been approved for the treatment of diabetic macular edema and neovascular age-related macular degeneration, and tarcocimab. RS
References
1. Ip M, Hendrick A. Retinal vein occlusion review. Asia Pac J Ophthalmol (Phila). 2018;7:40-45.
2. Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014;132:13-22.
3. Scott IU, Campochiaro PA, Newman NJ, Biousse V. Retinal vascular occlusions. Lancet. 2020;396:1927-1940.
4. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336-339
5. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a Phase III study. Ophthalmology. 2010;117:1102-1112.
6. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133.
7. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: The 24-week results of the VIBRANT study. Ophthalmology. 2015;122:538-544.
8. Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS Study. Ophthalmology. 2014;121:1414-1420.
9. Clark WL, Boyer DS, Heier JS, et al. intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT Study. Ophthalmology. 2016;123:330-336.
10. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: One-year results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121:202-208.
11. Thach AB, Yau L, Hoang C, Tuomi L. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology. 2014;121:1059-1566.
12. Danzig CJ, Blotner S, Steffen V, Haskova Z. Clinical trial versus real-world outcomes with anti-VEGF therapy for branch and central retinal vein occlusion. Invest Ophthalmol Vis Sci. 2021;62:3174.
13. Callizo J, Ziemssen F, Bertelmann T, et al. Real-world data: ranibizumab treatment for retinal vein occlusion in the OCEAN Study. Clin Ophthalmol. 2019;13:2167-2179.
14. Pearce IA, Gupta AD, Lacey S, LUMINOUS. One-year outcomes of ranibizumab treatment in a cohort of patients with retinal vein occlusion: an interim analysis from the real-world LUMINOUS study. Invest Ophthalmol Vis Sci. 2015;56:3743-3743.
15. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802-809.
16. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion:12-month outcomes of a Phase III study. Ophthalmology. 2011;118:1594-1602.
17. Ho AC, Gray S, Rundle A, et al. Ranibizumab in patients with macular edema following retinal vein occlusion: 12-month outcomes of BRAVO and CRUISE. Invest Ophthalmol Vis Sci. 2010;51:6452.
18. Scott IU, Oden NL, VanVeldhuisen PC, Ip MS, Blodi BA, Chan CK. Month 24 outcomes after treatment initiation with anti-vascular endothelial growth factor therapy for macular edema due to central retinal or hemiretinal vein occlusion: SCORE2 Report 10: A secondary analysis of the SCORE2 randomized clinical trial. JAMA Ophthalmol. 2019;137:1389-1398.
19. Ip MS, Oden NL, Scott IU, et al. Month 12 outcomes after treatment change at month 6 among poor responders to aflibercept or bevacizumab in eyes with macular edema secondary to central or hemiretinal vein occlusion: A secondary analysis of the SCORE2 Study. JAMA Ophthalmol. 2019;137:281-287.
20. Larsen M, Waldstein SM, Boscia F, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: Twelve-month results of the CRYSTAL Study. Ophthalmology. 2016;123:1101-1011.
21. A study to evaluate the efficacy and safety of faricimab in participants with macular edema secondary to branch retinal vein occlusion (BALATON). ClinicalTrials.gov identifier NCT04740905. Updated September 6, 2022. https://clinicaltrials.gov/ct2/show/NCT04740905
22. A Study to evaluate the efficacy and safety of faricimab in participants with macular edema secondary to central retinal or hemiretinal vein occlusion. ClinicalTrials.gov Identifier NCT04740931. Updated August 31, 2022. https://clinicaltrials.gov/ct2/show/NCT04740931?term=faricimab&draw=2&rank=5
23. A Study to Evaluate the Efficacy, Durability, and Safety of KSI-301 Compared to Aflibercept in Patients With Macular Edema Due to Retinal Vein Occlusion (RVO). ClinicalTrials.gov Identifier NCT04592419. Updated July 22, 2022. https://clinicaltrials.gov/ct2/show/NCT04592419
24. Singer M. KSI-301 anti-VEGF antibody biopolymer conjugate for retinal vein occlusion: primary 24-week efficacy and safety outcomes of the BEACON Phase 3 Pivotal Study. Abstract presented at the American Academy of Ophthalmology 2022 Meeting, Retina Subspecialty Day; September 30, 2022; Chicago, IL.



