Take-home points
|
 |
|
Bio Dr. Finn is an DISCLOSURES: Dr. Finn disclosed relationships with Allergan/AbbVie, Genentech/Roche and Apellis Pharmaceuticals. |
The Retina Subspecialty Day sessions at the annual American Academy of Ophthalmology meeting are packed with important trial readouts and a host of presentations on new medical management and surgical techniques. This year’s meeting in Chicago lived up to expectations.
Here we present four notable abstracts from the meeting:
- a literature review of outcomes for internal limited membrane peeling in patients with diabetic macular edema;
- an update from the TRUCKEE study of real-world outcomes in patients receiving faricimab for neovascular age-related macular degeneration;
- 48-month results from the PULSAR study of high-dose aflibercept for patients with nAMD; and
- an update from the DRCR Retina Network Protocol AC trial of patients randomized to aflibercept monotherapy or bevacizumab first.
Is ILM necessary in diabetic vitrectomy?
 |
Internal limiting membrane peeling in diabetic vitrectomy has been proposed for DME, proliferative diabetic retinopathy and tractional retinal detachment. However, we don’t fully understand the long-term effects or even immediate benefits of ILM peeling.
Stanley Chang, MD, of the Edward Harkness Eye Institute at Columbia University Medical Center in New York, described the important role Müller cells play as the primary glial cells of the retina.1 ILM peeling injures Müller cells and nerve fiber layer axons. Müller cells are important in maintaining retinal function including recycling neurotransmitters, preventing glutamate toxicity, participating in the retinoid cycle, and regulating nutrient supply and blood flow to the retina. Disturbing the Müller cells can affect all the cells in the retina.
In diabetes, hyperglycemia activates Müller cells, increasing cytokine and chemokine release. The retinal thickness is also decreased in patients with severe diabetic eye disease.
Dr. Chang performed a literature review that found no difference in best corrected visual acuity, postoperative central macular thickness or macular thickness reduction in patients undergoing ILM peeling for DME.
ILM peeling in PDR showed slightly more promising results. Patients undergoing ILM peeling for PDR or TRD had better BCVA, needed fewer anti-VEGF injections, and had a lower postoperative rate of DME and epiretinal membrane formation. Dr. Chang noted some important limitations, namely that VA results for patients undergoing subsequent epiretinal membrane peeling weren’t available.
Dr. Chang reviewed his own cases, noting that the ILM shouldn’t be peeled if it appears normal. No strong clinical evidence exists that peeling ILM in cases of DME leads to better outcomes. He advised peeling ILM in diabetic eyes with macular traction if striae are present and underscored that we don’t know the long-term effects of ILM peeling on DR, especially in younger patients.
Dr. Chang disclosed relationships with Alcon Laboratories and Genentech.
Update from TRUCKEE study of faricimab
 |
TRUCKEE is an ongoing collaborative clinician-run trial across the United States looking at real-world outcomes in patients receiving faricimab (Vabysmo, Genentech/Roche).2 So far, 491 patients with nAMD have been enrolled, receiving a total of 1,231 injections; follow-up data are available on 335 patients. The majority were high-need patients and most were switched from aflibercept (Eylea, Regeneron Pharmaceuticals) to faricimab.
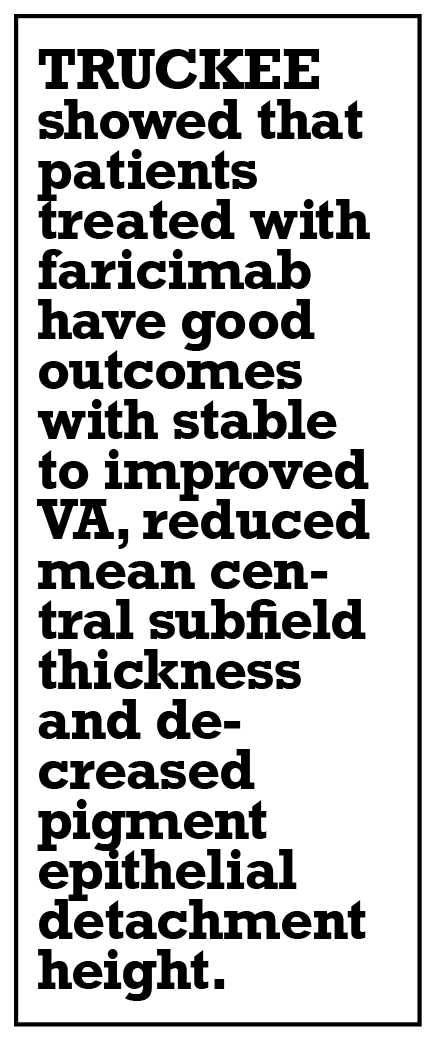
Overall, TRUCKEE showed that patients treated with faricimab have good outcomes with stable to improved VA, reduced mean central subfield thickness and decreased pigment epithelial detachment height.
Treatment-naive patients gained on average 4.9 letters, had an 84.5-µm reduction in mean CST and 93-µm reduction in PED height. After one faricimab injection, 40 percent of patients had resolution of intraretinal fluid, 25 percent had total resolution of subretinal fluid and 41.7 percent had total resolution of PEDs.
 |
For all patients switched from any anti-VEGF medication to faricimab, IRF resolution occurred in 17.8 percent of eyes, SRF resolution in 36.6 percent and PED resolved in 11.1 percent. For those eyes switched from aflibercept, 12.3 percent had complete IRF resolution, 37.2 percent complete SRF resolution and 3.2 percent complete PED resolution. All switched patients maintained or extended their prior-treatment interval.
Faricimab was well-tolerated with one case of infectious endophthalmitis and one case of intraocular inflammation in the study. Ramanath Bhandari, MD, of Spring Clinic Eye Institute in Springfield, Illinois, was asked about the need for repeating loading doses when switching a patient to faricimab and advised that a loading dose is beneficial for patients that are amenable when switching therapies.
Dr. Bhandari disclosed relationships with Apellis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, RevOpsis and Vial.
High-dose aflibercept extends intervals
 |
PULSAR was a multicenter, randomized, double masked study of 1,011 patients with treatment-naive nAMD.3 Patients were randomized 1:1:1 to aflibercept 2 mg q8 weeks, aflibercept 8 mg q12 weeks or aflibercept 8 mg q16 weeks, all after three monthly injections. Baseline patient characteristics were similar across all groups and the study met its primary endpoint at week 48.
At 48 weeks, the standard dosing group gained 6.2 letters, the 8-mg/q12-weeks group gained 6.7 letters and the 8-mg/q16-weeks group gained 7.6 letters, Paolo Lanzetta, MD, of the University of Udine and the European Institute of Ocular Microsurgery in Milan, reported.
At week 16, 63 percent of patients on 8-mg treatment didn’t have any retinal fluid compared to 52 percent in the 2-mg group. Overall, 83 percent of patients receiving the 8-mg dose maintained a greater-than-q12-weeks dosing. Seventy-nine percent of patients maintained a q12-week regimen and 77 percent a q16-week dosing.
All three groups had a similar CRT, and no group saw a significant see-sawing effect over the 48 weeks. The higher dose was well tolerated and no significant safety signals were reported during the study.
Importantly, intraocular pressure increase was similar across all groups, with a 2.1 percent rise in the 2-mg group and a 3 percent increase in the 8-mg groups. PULSAR shows some encouraging results for increasing duration with high dose aflibercept.
Dr. Lanzetta disclosed relationships with AbbVie, Aerie Pharmaceuticals, Apellis Pharmaceuticals, Bausch + Lomb, Bayer Healthcare Pharmaceuticals, Biogen, Boehringer Ingelheim, Centervue, Genentech, Novartis, Outlook Therapeutics and Roche.
 |
Aflibercept vs. bevacizumab for DME
The Protocol AC study of the DRCR Retina Network evaluated aflibercept monotherapy or bevacizumab (Avastin, Roche/Genentech) first for diabetic macular edema.4,5
We know from Protocol T that patients with 20/50 or worst vision who were started on aflibercept did better at one and two years.6 Insurers are increasingly demanding that we employ “step therapy” to reduce costs, but we don’t know if this treatment strategy compromises visual outcomes for patients.
 |
In Protocol AC, patients with 20/50 or worse vision were randomized to aflibercept monotherapy or bevacizumab first. At 12 weeks, patients receiving bevacizumab first could be switched to aflibercept based on the following criteria: persistent DME; injection with bevacizumab at the last two visits; no recent improvement; and continued suboptimal vision (20/50 or worse before 24 weeks and 20/32 or worse after 24 weeks). Once patients were switched, they were treated with aflibercept for two monthly visits then retreated according to preset criteria.
The bevacizumab-first patients averaged 1.5 more injections over two years. Over two years, 70 percent of patients switched from bevacizumab first to aflibercept. No overall difference in mean vision or retinal thickness was found between the two groups. The number of patients with two- and three-line improvement were similar in both groups. CST wasn’t statistically significantly different between the two groups, although the results slightly favored aflibercept.
At two years, 62 percent of aflibercept eyes and 55 percent of bevacizumab eyes had complete DME resolution. Adverse events were similar across both groups.
Chirag Jhaveri, MD, of the Retina Consultants of Austin and the Austin Research Center for Retina in Texas, concluded that rescue treatment with aflibercept mitigated the average visual and anatomic difference that arose from initiating therapy with bevacizumab versus aflibercept. Initiating treatment with bevacizumab may result in cost reductions for the health-care system without significant difference in visual outcomes over two years.
Dr. Jhaveri disclosed relationships with Boehringer Ingelheim, Genentech/Roche, Gyroscope Therapeutics, Kodiak Sciences, Novartis, Opthea, Oxurion and RegenxBi. RS
REFERENCES
1. Chang S. Is it necessary to peel internal limiting membrane in diabetic vitrectomy? Paper presented at American Academy of Ophthalmology Retina Subspecialty Day, Session RET02; Chicago, IL; September 30, 2022.
2. Bhandari R. Real world efficacy, durability and safety of faricimab in neovascular age-related macular degeneration: TRUCKEE study. Paper presented at AAO Retina Subspecialty Day, Session RET09; Chicago, IL; September 30, 2022.
3. Lanzetta P. Intravitreal aflibercept injection 8 mg for neovascular age-related macular degeneration: 48-week results from the Phase 3 PULSAR trial. Paper presented at AAO Retina Subspecialty Day, Session RET09; Chicago, IL; September 30, 2022.
4. Jhaveri CD. DRCR Retina Network: Protocol AC results. Paper presented at AAO Retina Subspecialty Day, Session RET10; Chicago, IL; October 1, 2022.
5. Jhaveri CD, Glassman AR, Ferris FL 3rd, et al; DRCR Retina Network. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387:692-703.
6. Bressler NM, Beaulieu WT, Maguire MG, et al; Diabetic Retinopathy Clinical Research Network. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am J Ophthalmol. 2018;195:93-100.



