 |
There are no guidelines from the American Academy of Ophthalmology or Medicare regarding the indications for macular surgery for vitreomacular interface disorders, so the decision for treatment ultimately rests with the surgeon’s best judgment and experience.5
There are many variations in membrane peeling techniques, including the use and choice of biologic stains, peeling of the internal limiting membrane (ILM) and the tools or techniques used to accomplish these maneuvers. Controversies over these are beyond the scope of this article and reflect surgeon preference more than science.
With that in mind, I humbly present my preferred technique for macular surgery—not as science or dogma, but as real-world knowledge accumulated from successes and failures, intended to help you avoid “teachable moments.” Regardless of preferences, an agreeable goal for macular surgery is to relieve symptomatic macular distortion without iatrogenic damage to the macula.
Surgical Approach and Prep
Macular surgery can be intimidating to new vitreoretinal surgeons given the fragility of the retina to manipulation and the importance of the macula to functional and occupational vision. The vitreous, retina and often the abnormal epiretinal tissue are optically clear, which adds significant challenge to initiating membrane peeling and determining an endpoint.
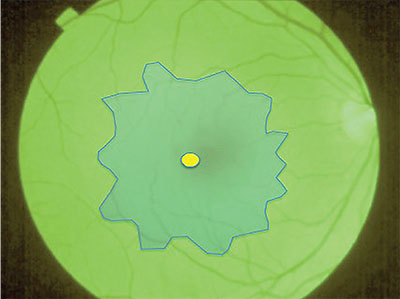 |
| Figure 1. With infusion pressure lowered, apply “heavy” indocyanine green stain to cover the entire macula. Allow it to settle for 10 seconds then remove, leaving a robust green staining of the internal limiting membrane and negative staining of the epiretinal membrane complex. |
Here, I present a simple technique that is effective for removal of most epiretinal membranes. It utilizes the advantages of a biologic stain, “heavy” indocyanine green (ICG) diluted in 5% dextrose solution, to differentiate normal from abnormal structures.6-9 My preferred technique is to peel the ILM in all cases, lifting the epiretinal membrane (ERM) with the underlying ILM scaffold and removing the substrate for persistent or recurrent epiretinal membranes. Complete ILM peeling of the macula is not safe in all cases, and is not mandatory for visual gains.10
Critical steps for planning surgery include evaluating the status of the posterior hyaloid face and the configuration of the tractional complex with funduscopy and spectral domain optical coherence tomography.11-14 Although most idiopathic ERMs occur after a posterior vitreous detachment (PVD), vitreoschisis (splitting of the posterior hyaloid face into layers) with a retained layer of posterior cortical vitreous covering the retina commonly accompanies secondary causes of vitreomacular interface disorders, particularly vascular and inflammatory diseases, or with chronic macular hole.15-19
Applying Biologic Stains
After core vitrectomy, elevation of the posterior hyaloid face is required to apply biologic stains to the ERM or ILM and to gain access with instruments for membrane peeling. SD-OCT can identify areas of vitreous attachment, vitreous traction and surgical planes between the epiretinal tissue and the inner retina. Aspiration with the vitreous cutter just over the optic disc with or without diluted triamcinolone acetonide stain can induce a PVD.
SD-OCT can also provide information about the character and anticipated surgical behavior of the ERM. For a diffuse, sheet-like ERM with uniform thickness that extends beyond the boundary of a standard 6-mm horizontal raster scan, the membrane is often elastic, cohesive and stains poorly with both triamcinolone and “heavy” ICG. This type of ERM commonly appears in patients with a history of diabetic retinopathy or posterior staphyloma from pathologic myopia.
In eyes with a history of uveitis, the ERM and ILM are often friable. Attempted ILM peeling may require multiple regrasps and can induce iatrogenic macular trauma. Cases with vitreomacular traction or macular hole on OCT typically have strong adhesions at the foveal Müller cell cone. You must use caution when peeling across the fovea in such cases, as iatrogenic traction could induce or enlarge a full-thickness macular hole.
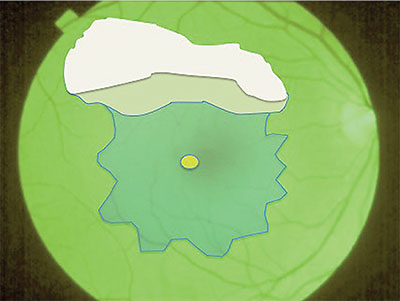 |
| Figure 2. To initiate peeling of the internal limiting membrane, create a horizontal contiguous row of breaks in the ILM with a scraper, and use ILM forceps to advance the edge of elevated ILM to the border of the epiretinal membrane. |
Preoperative disruption or disorganization of retinal lamination at the fovea by OCT is an important prognostic indicator for limited visual improvement despite relief of vitreomacular interface disease.13
The Surgical Technique
• Step 1: Apply ‘Heavy’ ICG Stain. After performing core vitrectomy and confirming the elevation of the posterior hyaloid face, apply approximately 0.3 mL of “heavy” ICG for 10 seconds over the macula without a fluid-air exchange (Figure 1). The volume injected should be sufficient to cover the macula as a confluent puddle.
Three techniques can facilitate stasis of the ICG stain over the macula: using valved cannulas; reducing infusion pressure; or suspending the automatic intraocular pressure-regulating feature on the vitrectomy system. Avoid longer durations of ICG exposure to reduce the risk of ICG toxicity.20-25 Prolonged exposure also increases fragility of the ILM, resulting in shearing of a brittle substrate into small fragments rather than the desired native elasticity that facilitates cohesion during peeling.
Once the ICG is removed, the ILM should stain moderately to intensely green; negative staining will reveal the ERM. If the ERM is diffuse or the plane between the membranes and inner retina is minimal on SD-OCT, ICG staining of the underlying ILM may be more patchy. An absence of initial staining indicates a diffuse ERM that will require a pinch peeling technique; it is unlikely to cleave cleanly with a scraper.
• Step 2: Initiate ILM Peeling. Using a scraper or forceps, fracture the ILM at a natural weak point overlying the large retinal vessels, at the inferior arcade. Angle conformal ILM forceps most tangentially when reaching across the horizontal and vertical midlines from their respective sclerotomy. This helps to prevent an asymmetric, deep grab that could result in bleeding or trauma to the inner retina.
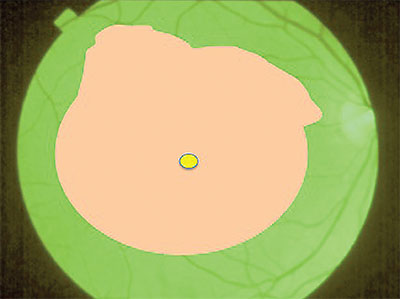 |
| Figure 3. Peel the internal limiting membrane and epiretinal membrane complex together, removing the complex with forceps as a single sheet to release all traction from the macula. |
My preferred technique is to scratch toward my instrument’s sclerotomy with a diamond-dusted membrane scraper or nitinol loop to create a series of contiguous horizontal edges of elevated ILM for a broad plane to continue the peel. This allows multiple opportunities for regrasping with ILM forceps if the ILM shears at any point.
Initiating the ILM peel distant to the fovea affords room for regrasping without risk of traumatizing the fovea, if the ILM shears posteriorly. Peel the ILM to the boundary of the ERM, trying to keep as broad an edge as possible. Keep the forceps close to the retinal surface when propagating the peel to prevent narrowing of the elevated band by shearing. Remember, the macula is a concave structure, except where there is traction or edema. Try not to amputate the peeled flap of stained ILM in order to preserve landmarks for regrasping.
• Step 3: Peel the ILM and ERM Complex Together. Once the ILM peel reaches the boundary of the ERM, release and fold the peeled ILM edge over to expose the base of the ERM to grasp the ERM and ILM together (Figure 2, page 29). In eyes with diffuse macular ICG staining, which occurs with thin membranes and those with a prominent cleavage plane on SD-OCT, you may continue the peel through the fovea without specifically regrasping to include the ERM.
Closing the forceps to sweep and then pin the edge is an effective technique to control the floating peeled tissue that otherwise is subject to movement from the fluidics of the infusion. I prefer to reduce infusion pressure and turn off the automated intraocular pressure control feature during membrane peeling to reduce this movement. A higher infusion pressure may be preferred in eyes with a risk for bleeding from retinal neovascularization as part of the ERM complex.
 |
I prefer to peel through the fovea and nasal macula first to release traction from areas most likely to be producing the patient’s visual complaints and to avoid regrasps that could traumatize these areas later in the peeling. If there is strong adhesion at the fovea, I peel circumferentially around the fovea before removing a plume of epifoveal tissue.
Again, try to sweep slowly with the forceps shallowly over the retinal surface to radiate and broaden the peel rather than shear or narrow it. If the vertically oriented strip amputates, it will leave two planes for circumferential peeling.
It’s important to reorient the forceps perpendicular to the new edge and grasp as peripherally as possible to make the peel broad and avoid regrasps that would threaten the fovea. This technique closely resembles propagation of an anterior capsulorhexis and has been coined a “maculorhexis.” If all stained landmarks have been lost, restaining with ICG may reveal new edges.
If the peeling requires multiple grasps, the ERM and ILM may cleave into separate planes. Restaining can reveal ILM remnants if you desire complete ILM peeling in the rhexis. Limit exposure times with ICG to avoid toxicity.
Most importantly, the goals of macular surgery should be at the forefront during peeling: 1) relieve traction from the fovea; and 2) avoid traumatizing the macula. Therefore, the surgeon should aim for good, not perfect, and avoid picking excessively at peripheral ERM or ILM remnants after accomplishing the primary goal of surgery.
Conclusion
Biologic stains enhance the visibility of normal and pathologic structures during macular surgery. This may improve safety by limiting manipulation of normal tissue, which is critical because the retina is inherently fragile.
| Take-home Point In the absence of guidelines for macular surgery for vitreomacular interface disorders, the surgeon must rely on his or her best judgment and experience for treatment. This article describes a technique for membrane peeling that uses indocyanine green biologic stain and peeling of the internal limiting membrane and epiretinal membrane complex together. |
The simple technique I’ve outlined here is effective for removing most ERMs and can be applied with any gauge surgery and with any vitrectomy system. We have not experienced safety issues with “heavy” ICG at our institution, and it has been cost-effective for our surgery center. RS
REFERENCES
1. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403-425; discussion 425-430.
2. Meuer SM, Myers CE, Klein BE, et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The Beaver Dam Eye Study. Ophthalmology. 2015;122:787-795.
3. Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110:34-40.
4. Bu SC, Kuijer R, Li XR, et al. Idiopathic epiretinal membrane. Retina 2014;34:2317-2335.
5. Idiopathic epiretinal membrane and vitreomacular traction preferred practice pattern–2015. American Academy of Ophthalmology website. Available at: http://www.aao.org/preferred-practice-pattern/idiopathic-epiretinal-membrane-vitreomacular-tract. Updated November 2015. Accessed October 10, 2016.
6. Burk SE, Da Mata AP, Snyder ME, Rosa RH Jr, Foster RE. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology 2000;107:2010-2014.
7. Da Mata AP, Burk SE, Riemann CD, et at. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology. 2001;108:1187-1192.
8. Foster RE, Petersen MR, Da Mata AP, Burk SE, Rosa RH Jr, Riemann CD. Negative indocyanine green staining of epiretinal membranes. Retina. 2002;22:106-108.
9. Kaehr MM, Apte RS. Combined epiretinal and internal limiting membrane peeling facilitated by high dilution indocyanine green negative staining. J Ophthalmic Vis Res. 2015;10:495-497
10. Liu H, Zuo S, Ding C, Dai X, Zhu X. Comparison of the effectiveness of pars plana vitrectomy with and without internal limiting membrane peeling for idiopathic retinal membrane removal: A meta-analysis. J Ophthalmol. Epub 2015 Nov 26.
11. Watanabe A, Arimoto S, Nishi O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings. Ophthalmology. 2009;116:1788-1793.
12. Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016;10:527-534.
13. Scheerlinck LM, van der Valk R, van Leeuwen R. Predictive factors for postoperative visual acuity in idiopathic epiretinal membrane: a systematic review. Acta Ophthalmol. 2015;93:203-212.
14. Kim HJ, Kang JW, Chung H, Kim HC. Correlation of foveal photoreceptor integrity with visual outcome in idiopathic epiretinal membrane. Curr Eye Res. 2014;39:626-633.
15. Wise GN. Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol 1975;79:349-347.
16. Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16:416-22.
17. Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9:253-260.
18. Shinoda K, Hirakata A, Hida T, et al. Ultrastructural and immunohistochemical findings in five patients with vitreomacular traction syndrome. Retina 2000;20:289-293.
19. Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol 2002;86:902-909.
20. Kwok AK, Lai TY, Yew DT, Li WW. Internal limiting membrane staining with various concentrations of indocyanine green dye under air in macular surgeries. Am J Ophthalmol. 2003;136:223-230.
21. Haritoglou C, Gandorfer A, Gass CA, Kampik A. Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004;137:345-348.
22. Lai CC, Wu WC, Chuang LH, Yeung L, Chen TL, Lin KK. Prevention of indocyanine green toxicity on retinal pigment epithelium with whole blood in stain-assisted macular hole surgery. Ophthalmology. 2005;112:1409-1414.
23. Gandorfer A, Haritoglou C, Gandorfer A, Kampik A. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003;44:316-323.
24. Haritoglou C, Priglinger S, Gandorfer A, Welge-Lussen U, Kampik A. Histology of the vitreoretinal interface after indocyanine green staining of the ILM, with illumination using a halogen and xenon light source. Invest Ophthalmol Vis Sci. 2005;46:1468-1472.
25. Rodrigues EB, Meyer CH, Mennel S, Farah ME. Mechanisms of intravitreal toxicity of indocyanine green dye: implications for chromovitrectomy. Retina. 2007;27:958-970.



