| By Richard Mark Kirkner, Editor |
 |
Clinical programs for the treatment of geographic atrophy have taken on an almost Holy Grail-type mystique. At least 10 candidates targeting different pathways to treat the end-stage effects of dry age-related macular degeneration were in human trials at the start of the year.
A San Francisco Bay area company is pursuing a different approach for GA. At the International Society of Stem Cell Research 2021 meeting in June, Jane Lebkowski, PhD, president of Regenerative Patch Technologies, reported results of the Phase I/IIa trial of its CPCB-RPE1 implant (NCT01590692) for treatment of severe vision loss from GA.
CPCB-RPE1 is a bioengineered implant consisting of stem cell-derived, mature, polarized retinal pigment epithelial cells on what the company describes as an “ultrathin” synthetic parylene membrane. It’s placed in a subretinal bleb overlying the area of GA to replace damaged RPE and Bruch’s membrane.
Here, Amir H. Kashani, MD, PhD, associate professor of ophthalmology at Wilmer Eye Institute, Johns Hopkins University in Baltimore, answers questions about the technology and the clinical investigation. Dr. Kashani is the lead trial investigator for the Phase I/IIa study and was formerly on faculty at the University of Southern California, which is one of three entities—California Institute of Technology and University of California Santa Barbara are the others—that have licensed the technology to RPT. He has no financial interest in the company or technology, but USC has received grant support from RPT for conducting the clinical trial.
Q: Please describe the CPCB-RPE1 implant in your own words.
A: This biosynthetic implant consists of an RPE monolayer derived from stem cells and adherent to a synthetic parylene membrane that mimics the diffusion properties of Bruch’s membrane. The implant is approximately 3.5-x-6.25-mm in area and 6 µm thick with ultrathin regions less than 1 µm thick. It’s designed to be delivered into the subretinal space and within the area of GA in an outpatient procedure.
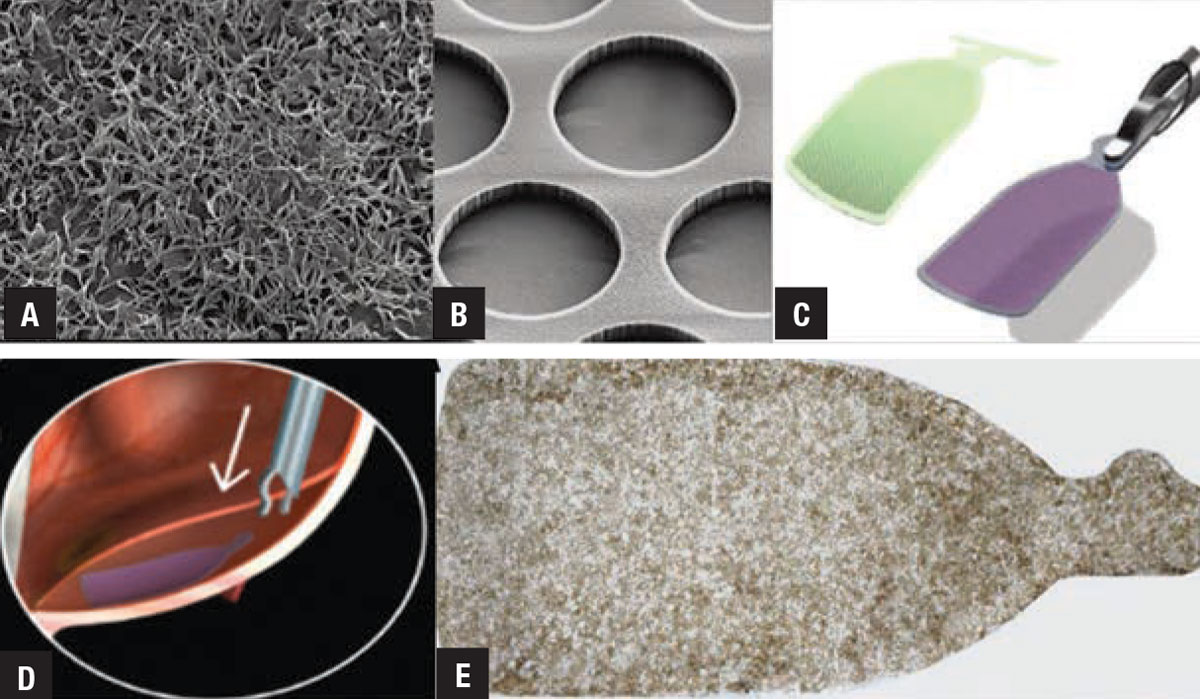 |
| Five views of the CPCB-RPE1 implant: A) mature retinal pigment epithelium cells on the implant; B) the parylene membranes used as a scaffold to support the RPE cells; C) a rendering of the delivery tool along with the implant showing how the tools grasp and compact the implant for delivery; D) a rendering of implant delivery into the subretinal space of the eye; E) a magnified view of the actual implant. (Image courtesy of Regenerative Patch Technologies). |
Q: Where did the idea come from to use a stem-cell implant to treat GA?
A: Over the past several decades a number of clinical trials attempted either macular translocation surgery or autologous RPE transplantation in patients with both wet and dry AMD. And the question was, knowing that GA involves an area of the retina where RPE cells are dying or dead, would it help people see if we put fresh or new RPE cells in that area?
In the early days of stem-cell research, we didn’t have the technology to derive RPE cells from stem cells, so surgeons had to surgically harvest RPE cells from another part of the patient’s eye, or potentially from other sources, such as animals or human fetuses. However, those sources came with practical limitations as well as ethical issues that prevented widespread availability. Most importantly, surgically harvesting autologous RPE was a traumatic process that often caused proliferative vitreoretinopathy.
Nevertheless, autologous RPE transplantation was done in several studies. Even though these surgeries were complicated and difficult, a handful of patients showed promising visual acuity improvements. They provided a proof-of-concept that RPE replacement can work.
Q: How does the parylene membrane help to integrate with the host tissue?
A: Parylene is a bioinert substance with a US Pharmacopeia Class VI rating for medical grade safety. It has been used for decades in many human device implants because it doesn’t elicit an inflammatory response, and it doesn’t degrade or dissolve. There’s extensive knowledge about its safety and biocompatibility.
 |
Engineers at USC and Caltech have been able to machine parylene down so that the CPCB-RPE1 implant has areas of parylene that are less than 1 µm thick. In-vitro studies have shown that a number of macromolecules that would theoretically diffuse through Bruch’s membrane can also diffuse through the ultrathin regions of the parylene membrane.
Q: What were the results of the Phase I/IIa trial?
A: Fifteen patients were implanted with the CPCB-RPE1 during outpatient surgery. Preliminary results of the first four patients to receive the implant were reported in 20181 and detailed methods of the surgery were described in a subsequent paper in 2020.2
These studies demonstrated a few important points. First, it was feasible to do the surgery with commercially available surgical instrumentation. Second, the success of the surgical procedure in targeting the area of GA was very high. Third, the implant appeared to retain viable RPE throughout the post-implantation period without clinical evidence of immune rejection.
Most importantly our recent results demonstrated that the implant and surgery are safe and well tolerated out to one year.3 We also observed that more eyes that received the implant gained vision while more non-implanted contralateral eyes tended to lose vision. This potential efficacy is promising but only preliminary and has to be verified in a subsequent clinical trial.
Q: Can you briefly describe the surgery itself?
A: The surgery is an outpatient procedure performed using commercially available vitrectomy equipment, including core vitrectomy, peripheral vitreous shaving and raising of a subretinal bleb in the peri-GA region.2 The delivery device is inserted through pars plana vitrectomy incisions and the implant is injected into the subretinal space through a retinotomy that’s about 1 mm wide.
After the implant is delivered into the subretinal space, the retina is flattened with perflurocarbon, air-fluid exchange and intraocular tamponade (silicone oil or gas).2 We used silicone oil for this pilot study because we were worried the implant may migrate, but that wasn’t a problem at all. We’re planning on using air or gas tamponade in our next trial which will also shorten the duration of surgery even more.
We used commercially available 23-gauge vitrectomy instrumentation for the entire procedure, except for the implant insertion which was done with the custom injector. RS
REFERENCES
1. Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097.
2. Kashani AH, Uang J, Mert M, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a Phase I/IIa study. Ophthalmol Retina. 2020;4:264-273.
3. Kashani AH, Lebkowski JS, Rahhal FM, et al. One year follow-up in a Phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Trans Vis Sci Tech. (In press).



