While topline data from the Phase IIb/III DAZZLE trial of the investigative anti-VEGF biopolymer conjugate KSI-301 failed to meet its primary endpoint of achieving noninferiority to aflibercept in neovascular age-related macular degeneration, leaders at trial sponsor Kodiak Sciences say they’re committed to pursuing the indication as well as other indications for the agent.
Retina specialists, meanwhile, have been parsing the data to make sense of why the 52-week results flopped.
 |
|
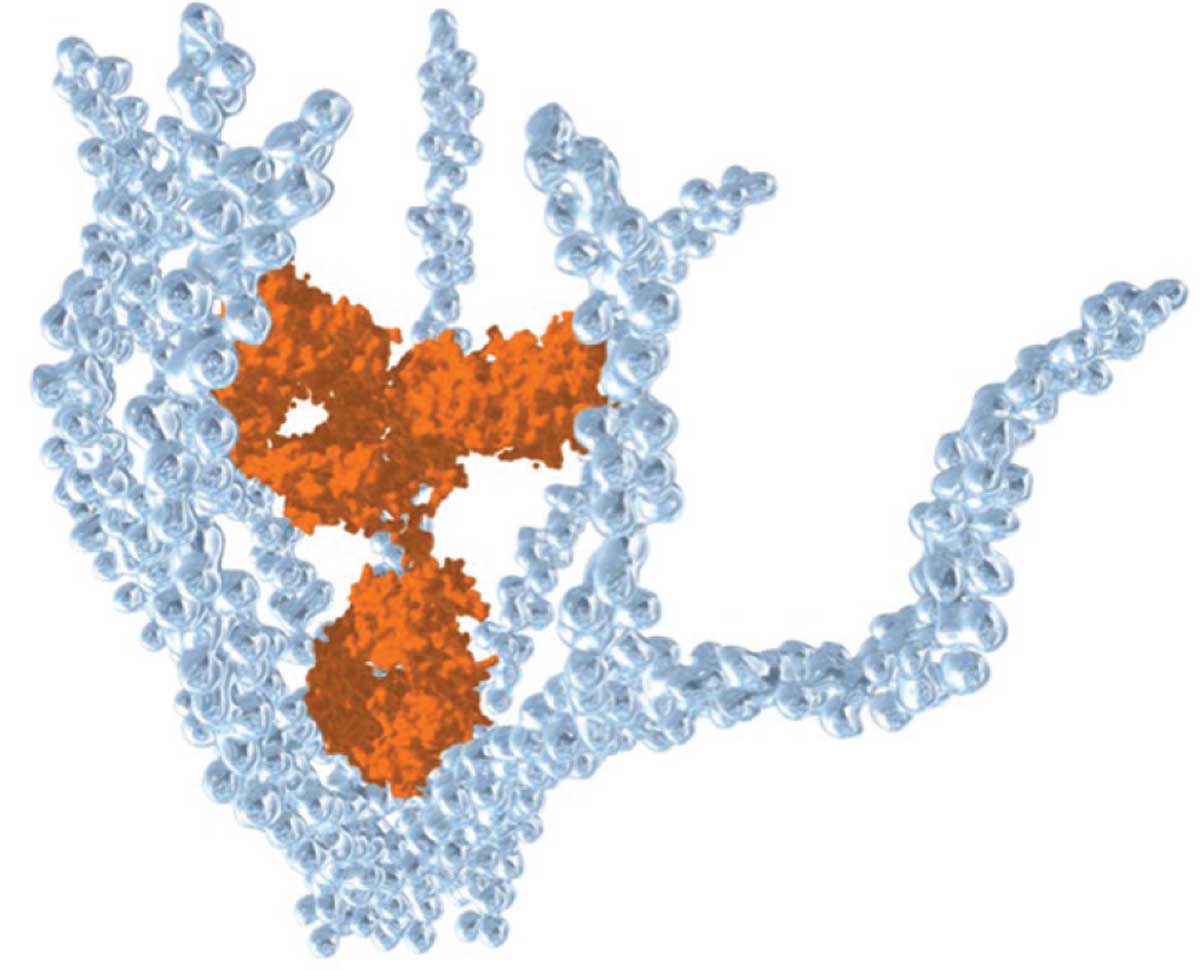
KSI-301 is an anti-VEGF antibody biopolymer conjugate of immunoglobin G1 antibody inert immune effector function (orange cluster) and branched high-molecular weight phosphorycholine polymer (light blue branches). (Courtesy Kodiak Sciences) |
“While we are highly disappointed that DAZZLE did not reach its primary endpoint, we do feel there are valuable insights into the potential for KSI- 301 and the value of the ABC (for anti-VEGF biopolymer conjugate) platform more broadly in the treatment of retinal disorders,” Kodiak Sciences chief executive officer Victor Perlroth, MD, told securities analysts in a conference call shortly after the results were reported.
Clinicians were likewise caught off guard by the results.
Stretched too far?
“I was surprised by the results of the IIb/III DAZZLE trial, especially the intraocular inflammation finding,” says David Eichenbaum, MD, a DAZZLE investigator and a vitreoretinal surgeon with Retina Vitreous Associates of Florida in the Tampa-St. Petersburg area.
DAZZLE randomized 559 patients to either KSI-301 5 mg or aflibercept 2 mg (Eylea, Regeneron Pharmaceuticals). KSI-301 patients were on a flexible treatment schedule of three, four or five months; the aflibercept group was on a fixed q8-week interval. Both groups received three monthly loading doses.
In previously reported comments, study investigator Carl Regillo, MD, of Wills Eye Hospital, Philadelphia, said that the three-month minimum for KSI-301 injections may have “stretched it too far” for the 30 percent of patients in that arm who could’ve benefited from more frequent treatments.
Says Dr. Eichenbaum, “The need for a significant minority of KSI-301 patients to receive treatment more often than every three months was less surprising to me, especially in the nAMD disease state under study in DAZZLE.”
Dr. Eichenbaum offers an explanation for why some patients in the KSI-301 arm may have needed more frequent dosing.
“The ABC platform is a large biopolymer, and nAMD is a purely subretinal disease,” he says. “Perhaps the pharmacokinetics and/or bioavailability of the anti-VEGF product had trouble penetrating subretinally; perhaps there’s just a proportion of nAMD patients with vascular endothelial growth factor load too great for the suppression offered by KSI-301 at q3 months.”
Overall topline results
At one year, the aflibercept arm had best-corrected visual acuity gains averaging 7 letters vs. 1 letter for the KSI-301 arm. As for anatomical outcomes, aflibercept showed average reductions in central subfield thickness of -133.9 µm vs. -91.5 µm with KSI-301.
The KSI-301 arm included three different treatment subgroups: 59.4 percent received q20-week treatment; 10.3 percent q16-week treatment; and 30.3 percent were on a q12-week interval. The q20-week group had an average 52-week BCVA improvement of 6.75 letters. The q12-week group dragged down the overall results, showing about a half-letter loss in BCVA.
Likewise, the q20-week KSI-301 group had anatomical outcomes more in line with the aflibercept group, with an average CST reduction around -100 µm. Again, the q12-week KSI-301 group didn’t show CST improvements as robust.
Safety outcomes
With regard to safety, KSI-301 had a 45.8 percent rate of treatment-emergent adverse events (TEAEs) in the treated eye vs. 36.4 percent with aflibercept.
Nine patients in the KSI-301 arm had at least one intraocular inflammation TAEA, a rate of 3.2 percent overall, while there were none in the aflibercept arm. IOI TAEAs included vitritis (3; 1.1 percent) and procedure-related endophthalmitis (1; 0.7 percent).
Dr. Eichenbaum notes that his practice’s trial site didn’t have any episodes of inflammation. “The aflibercept group performed remarkably well from a safety standpoint, with 0 percent IOI, emphasizing the safety of the well-developed Eylea product,” he says.
Ongoing trials
KSI-301 is the subject of five other Phase III trials: DAYLIGHT of monthly dosing in nAMD; BEACON of bimonthly treatment in retinal vein occlusion; GLEAM and GLIMMER of q2-to-q6-month dosing in diabetic macular edema; and GLOW of twice-yearly treatment in nonproliferative diabetic retinopathy. All but GLOW use aflibercept for the comparator: GLOW is using a sham injection.
Dr. Perlroth of Kodiak says topline data from GLEAM, GLIMMER and DAYLIGHT are expected in 2023.
Dr. Eichenbaum notes that RVO and DME “have a purely intraretinal pathophysiology, and perhaps the drug will perform well in a broader population at long intervals in those diseases. The designs for those trials are also more flexible for patients who may need treatment more often.”
With regard to safety, he says he’s “eager” to see results from DAYLIGHT. “I’m fairly certain that KSI-301 will perform well from an efficacy perspective at high-frequency dosing, and I am hopeful that the IOI rate will be improved, even at more frequent dosing, in this trial,” Dr. Eichenbaum says.
Besides serving as an investigator and consultant for Kodiak, Dr. Eichenbaum is also an investigator and consultant for Regeneron Pharmaceuticals. RS
— Richard Mark Kirkner
In BriefLumiThera, developer of the Valeda Light Delivery System photobiomodulation (PBM) treatment for retinal disease, has completed its acquisition of Diopsys and its electroretinography technology. LumiThera says the acquisition creates a complementary diagnosis and monitoring platform for its PBM system. Alimera Sciences reports positive three-year results of the PALADIN study of its Iluvien 0.19-mg sustained-release fluocinolone implant in diabetic macular edema. The results, published in Ophthalmology, Belgium-based ProQR Therapeutics reports that the pivotal Phase II/III Illuminate trial of sepofarsen for the treatment of CEP290-mediated Leber congenital amaurosis 10 didn’t meet its primary endpoint of vision improvement at 12 months. Stealth BioTherapeutics reports the final patient has completed treatment in the ReCLAIM-2 Phase II trial of elamipretide for extra-foveal geographic atrophy associated with dry age-related macular degeneration. Topline results are anticipated in the second quarter this year. |



