 |
Where ICGA is Helpful
In the past, we depended on fluorescein and indocyanine green angiography to identify and characterize these neovascular lesions, but over the past decade, optical coherence tomography has emerged as the principal imaging technique for patients with neovascular AMD.4
While OCT imaging can identify macular fluid in the different anatomic compartments of the macula and monitor fluid response to anti-VEGF therapy, at times it can be difficult to distinguish between macular fluid arising from neovascularization and that arising as a manifestation of non-vascularized AMD, such as subretinal fluid within the valley between drusen5 and the sub-RPE fluid that forms serous non-vascularized PEDs, which can also include pockets of subretinal fluid at the apex of the PED.6
ICGA can be helpful in identifying the neovascularization in PED if it exists.1 Often, type 1 neovascularization can be identified at the edge of the PED, or within a notch at the edge of the PED, and type 3 neovascularization can appear as a hot-spot within the retina on top of the PED. These type 3 lesions are usually also associated with intra-retinal fluid overlying the PED as well. But, what about those serous PEDs in which no neovascular lesion is obvious on angiography?
 |
Characteristics Of Non-vascularization
Over the years, we have identified some characteristic features on OCT that suggest when drusenoid or serous PEDs are not vascularized. If anti-VEGF therapy is initiated in the setting of a suspected non-vascularized lesion, and the fluid appears unresponsive to therapy, then it is perfectly reasonable to inject the patient and perform OCT imaging one-to-two weeks later, when the treatment response should be maximal, to look for a response.
If no response is seen, then the fluid probably is not dependent on vascular endothelial growth factor. If a response does occur, then the lesion is probably in an environment with an overabundance of VEGF, and monthly therapy is just inadequate to suppress the exudation.
Another strategy to use when the lesion appears unresponsive to anti-VEGF therapy, but the clinician is uncomfortable withdrawing therapy, is to slowly extend the interval and follow the lesion with OCT to determine if more fluid accumulates. The cases here demonstrate these management dilemmas.
Drusen, No Sign Of MNV
A 69-year-old woman was referred for a second opinion. She was originally diagnosed with neovascular AMD in the left eye and had received multiple anti-VEGF intravitreal injections, the last about six weeks
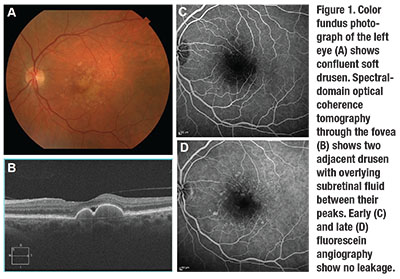
before her visit. Visual acuity was 20/25 in the left eye, and a fundus exam revealed confluent soft drusen (Figure 1).
SD-OCT imaging of the left eye showed two large drusen with subretinal fluid in between their peaks. However, FA did not show any leakage or sign of MNV. We made the decision to observe with serial SD-OCT imaging, and the subretinal fluid remained stable.
Non-vascularized Serous PED
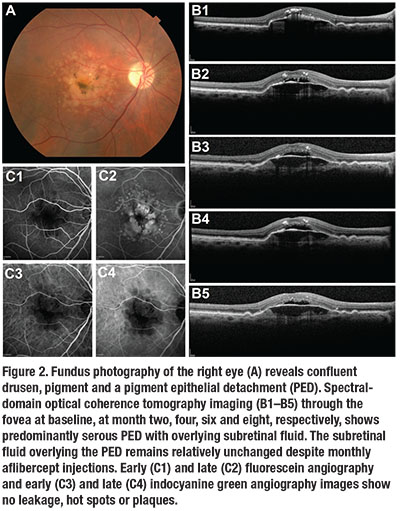
A 72-year-old man presented with blurred vision in his right eye. VA was 20/100 in the eye. A fundus exam revealed confluent drusen, pigment and a PED (Figure 2). SD-OCT imaging revealed a predominantly serous PED with overlying subretinal fluid.
He received monthly injections of aflibercept (Eylea, Regeneron) for the next seven months, during which the SD-OCT findings and VA remained unchanged. FA and ICGA did not show any sign of MNV. We stopped treatment and the lesion remained unchanged.
This lesion is characteristic of a non-vascularized, predominantly serous PED in AMD. Over time, these lesions will collapse into GA.
Overlying Subretinal Fluid
An 81-year-old woman, who was being followed for dry AMD in both eyes, presented with decreased vision in the left eye. VA was 20/30 in that eye. A fundus exam revealed a central PED with adjacent drusen (Figure 3, page 45). SD-OCT showed a predominantly serous PED with overlying subretinal fluid.
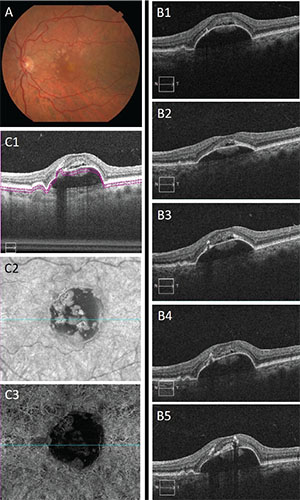 |
| Figure 3. Color fundus photography of the left eye (A) demonstrates central pigment epithelial detachment with adjacent drusen. Spectral-domain optical coherence tomography images at the fovea (B1–B5) at baseline, and months five (after five aflibercept injections), 13 (after three more injections), 20 (after two more injections) and 26 (after three more injections), respectively, show the size of the PED and overlying subretinal fluid remain relatively unchanged. SD-OCT angiography does not show any sign of neovascularization (C1-C3). |
‘Dry’ AMD a Misnomer?
These cases are all examples of subretinal fluid and drusen or PEDs in non-vascularized (“dry”) AMD unresponsive to anti-VEGF therapy. The question that arises in all of them is what is the source of the fluid in these lesions. The subretinal fluid is most likely the result of dysfunction of the RPE and its inability to pump fluid from the retina; the serous PEDs likely result from the fluid being pumped from the RPE and trapped within a lipid-laden, hydrophobic Bruch’s membrane. However, the exact mechanisms remain unproven.
 |
When subretinal fluid overlies the top of a PED, rather than on the edge of the PED, without associated intraretinal fluid, blood or a notch, then most likely the PED is not vascularized and will not respond to anti-VEGF therapy. Proceed with caution. RS
REFERENCES
1. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738.
2. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444.
3. Tan AC, Simhaee D, Balaratnasingam C, Dansingani KK, Yannuzzi LA. A perspective on the nature and frequency of pigment epithelial detachments. Am J Ophthalmol. 2016 Sep 13 [Epub ahead of print]
4. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration:
year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58.
5. Sikorski BL, Bukowska B, Kaluzny JJ, Szkulmowski M, Kowalczyk A, Wojtkowski M. Drusen with accompanying fluid underneath the sensory retina. Ophthalmology. 2011;118:82-92.
6. Madjarov B, Rosenfeld PJ. Serous PEDs with associated subretinal fluid unresponsive to anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2009;50:238.




