| UPDATE ON DRUG DELIVERY |
Take-home Points
|
 |
|
Bios Dr. Karl is a vitreoretinal surgery fellow at the Ohio State University Wexner Medical Center, Columbus. Dr. Ohr is a dual fellowship- Disclosures: Dr. Karl has no financial disclosures. Dr. Ohr has received research grants from Apellis, |
Anti-VEGF agents have revolutionized the treatment of neovascular age related macular degeneration, but, as retina specialists know all too well, real-world outcomes have fallen short of those seen in clinical trials in part due to the burden of frequent monitoring and treatment on patients and their families.1
Reducing this treatment burden and replicating the visual outcomes reported in clinical trials remains a major unmet need. Active areas of research to solve this problem include port delivery systems, high-molecular weight anti-VEGF antibody conjugates, subretinal gene therapy and others. Here, we review some potential future therapies that may mitigate or obviate the need for intravitreal injections in the future.
PDS with ranibizumab
Port Delivery System with ranibizumab (PDS, Genentech/Roche) is a permanent, refillable, surgically implanted drug delivery system that allows continuous delivery of a novel formulation of ranibizumab into the vitreous cavity by passive diffusion (Figure 1).2 The Food and Drug Administration last month accepted Genentech’s Biologics License Application under priority review for PDS, and issued an expected approval date by October 23.
In the Phase II Ladder clinical trial, 220 patients with newly diagnosed treatment-responsive nAMD were randomly assigned to treatment with the PDS with ranibizumab 10 mg/ml, 40 mg/ml, or 100 mg/ml, or to monthly intravitreal ranibizumab 0.5-mg injections (Lucentis,
Genentech/Roche).2
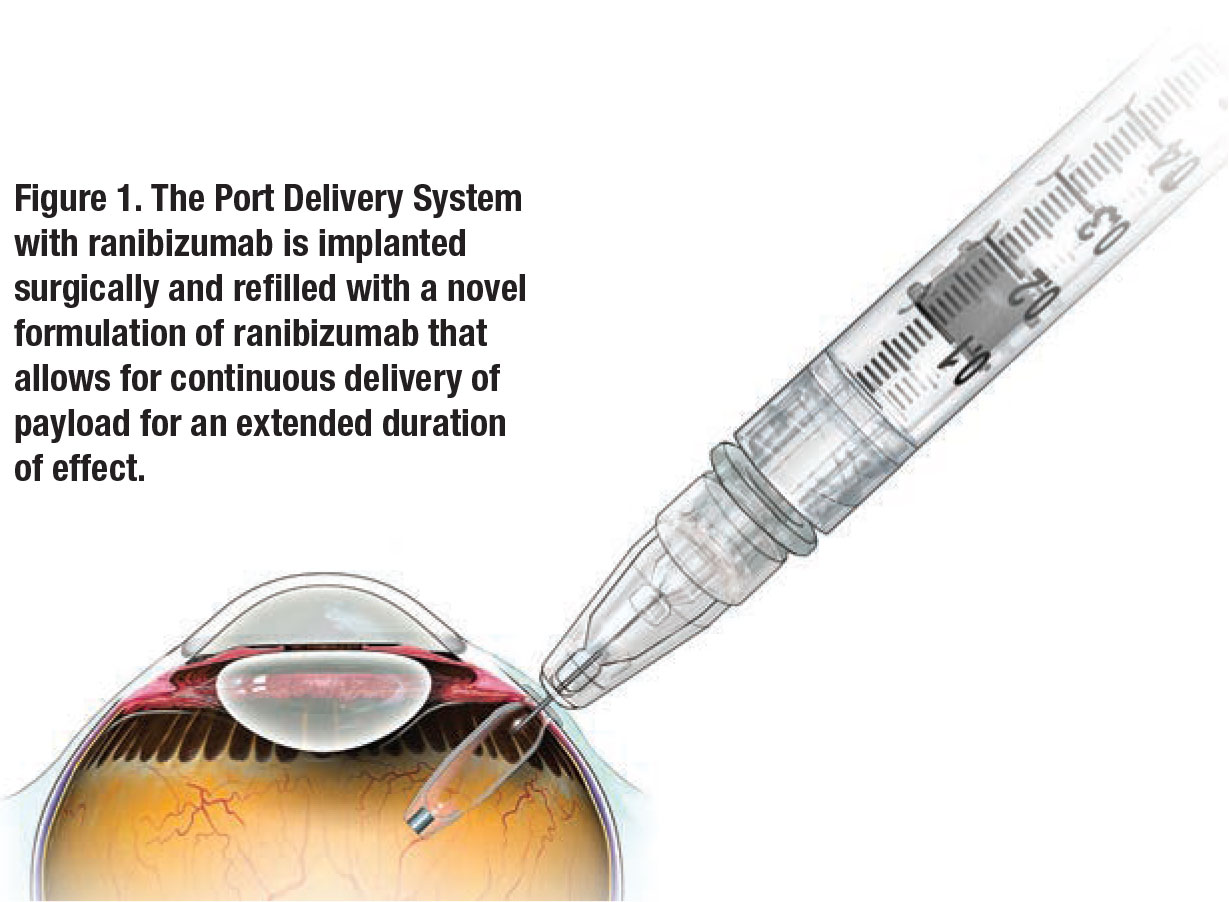
|
The primary efficacy endpoint was time to first implant refill. The 100-mg/ml PDS group demonstrated the longest time to implant refill—the median time for implant refill in the PDS 10-, 40 and 100-mg/mL arms was 8.7, 13 and 15.8 months, respectively—although the study wasn’t powered to detect a statistically significant difference between the 40- and 100-mg/mL arms.
The proportion of patients who didn’t require an implant refill for at least 12 months was 28.9, 56 and 59.4 percent in the PDS 10-, 40- and 100-mg/ml arms, respectively. Patients were treated with rescue therapy with ranibizumab 0.5 mg intravitreal injection in cases where predefined criteria weren’t met. Rescue therapy was required in 22.4, 4.8 and 1.7 percent of the 10-, 40- and 100-mg/mL arms, respectively.
Secondary Ladder Outcomes

|
Secondary efficacy outcomes included change in visual function and central foveal thickness. The adjusted best-corrected visual acuity from baseline at 22 months was −4.6, −2.3, +2.9 and +2.7 Early Treatment Diabetic Retinopathy Study letters for the 10-, 40- and 100-mg/mL PDS patients, and monthly 0.5-mg intravitreal ranibizumab injections patients, respectively. A post hoc analysis showed visual outcomes between the 100-mg/mL PDS group and the monthly 0.5-mg intravitreal ranibizumab group were comparable.
Importantly, the patients in this trial were noted to be responsive to anti-VEGF prior to enrollment, and so most had excellent vision at baseline. A dose response was similarly seen for anatomical outcomes. The adjusted change in central foveal thickness from baseline at 22 months was −0.7, −20.9, −4.0, and −10.9 µm for the 10-, 40- and 100-mg/mL PDS patients and monthly 0.5-mg intravitreal ranibizumab injections patients, respectively.
High rates of vitreous hemorrhage early in the trial initially prompted a pause in enrollment. Once the incision site at the pars plana was identified as the likely site of hemorrhage, the surgical procedure was modified to include laser ablation of the pars plana, reducing the incidence of vitreous hemorrhage from 50 to 5 percent.2
PDS Archway Trial

|
The Archway study is a Phase III randomized clinical trial of 418 patients comparing the PDS to ranibizumab. In the experimental arm, patients received ranibizumab delivered through the PDS implant with 100-mg/mL with refill-exchanges at fixed 24-week intervals. In the comparator arm, patients receive monthly injections of ranibizumab 0.5 mg. Initial results presented at the American Society of Retina Specialists annual meeting last year showed that 98.4 percent of PDS patients were able to last six months without needing additional injections and achieved visual acuity outcomes equivalent to patients receiving ranibizumab injections every four weeks.3
More details of the Phase III Archway trial of PDS with ranibizumab appear on page 29 in this issue.
The Portal study (NCT03683251) is an extension study of Ladder and Archway that will enroll 1,000 patients who will receive PDS 100 mg/mL with refill-exchanges administered every 24 or 36 weeks for approximately 144 weeks. The estimated study completion date is July 2026.
KSI-301
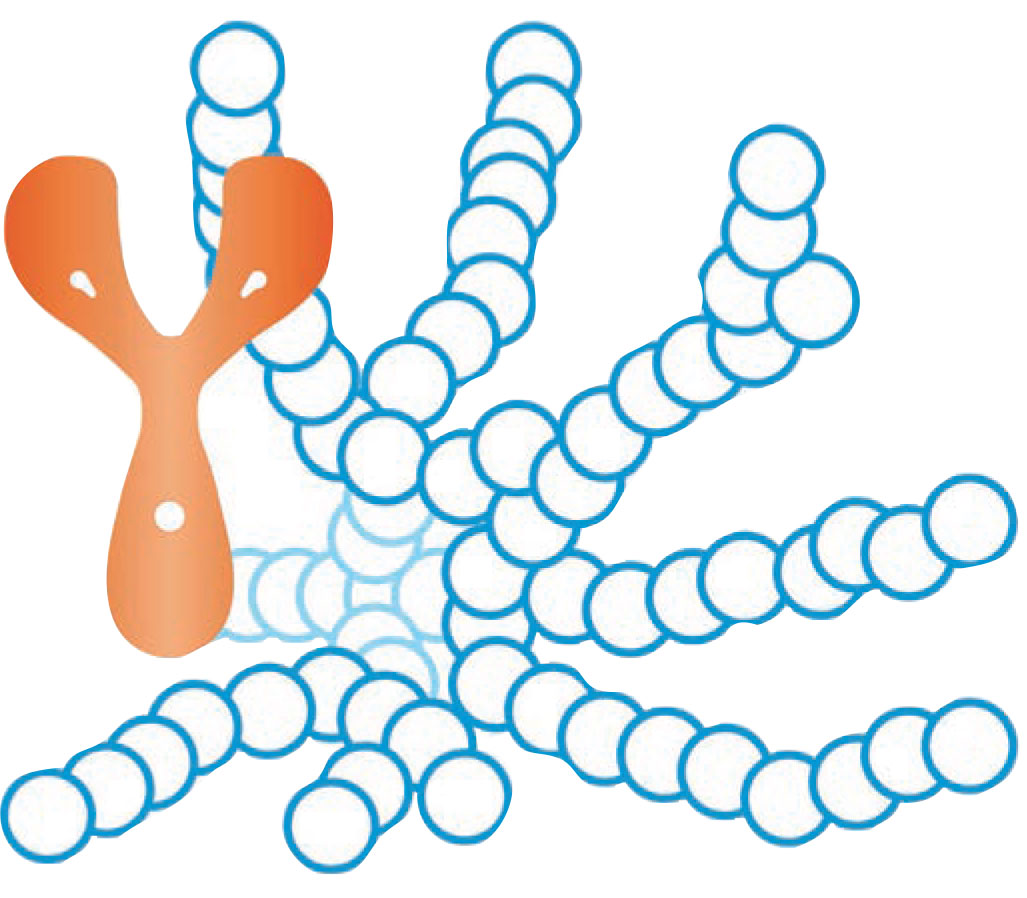
|
|
Figure 2. KSI-301 is a novel anti-VEGF biologic built on an antibody biopolymer conjugate that uses molecular weight to extend the durability of anti-VEGF effect. The Y-shaped neuron represents the antibody and the blue and white structure the biopolymer. |
While PDS provides continuous delivery of anti-VEGF, other methods of increasing the intraocular availability of anti-VEGF are being investigated. Large molecules are more stable and have longer intravitreal half-life.4 One such molecule, KSI-301 (Kodiak Sciences) is a novel anti-VEGF biologic built on an antibody biopolymer conjugate platform that’s designed to extend availability of the molecule in the eye by increasing its molecular weight (Figure 2).4,5 It’s administered as an intravitreal injection and is designed to provide sustained inhibition of VEGF for as long as six months.
DAZZLE (NCT04049266) is an ongoing Phase IIb/III randomized study designed to evaluate the efficacy, durability and safety of KSI-301 in patients with treatment-naïve nAMD. The trial enrolled 550 patients randomized 1:1 to receive either KSI-301 5 mg at 12, 16 or 20 months as specified in the study protocol, or aflibercept 2 mg once every four weeks for three months followed by once every eight weeks.
The primary outcome is mean BCVA and topline results are expected early next year. An earlier Phase I study (NCT03790852) resulted in visual improvements with a six-month interval of IVT in patients with nAMD along with other retinal vascular diseases.4
RGX-314
Subretinal gene therapy involves delivery of a viral vector to the subretinal space during vitrectomy. Once viral transduction occurs, the target host cells transcribe and translate the genetic material into proteins. Subretinal gene therapy has been shown to be effective in the treatment of RPE65-mediated inherited retinal dystrophy, for which voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics) received FDA approval.6 Gene therapies are currently in development for choroideremia and X-linked retinoschisis.
RGX-314 (RegenxBio) is a recombinant adeno-associated virus (AAV) gene therapy vector carrying a coding sequence for a soluble anti-VEGF protein (Figure 3, below). Delivery of this protein into the subretinal space during vitrectomy as a one-time therapy could potentially reduce the treatment burden of currently available therapies while maintaining vision.
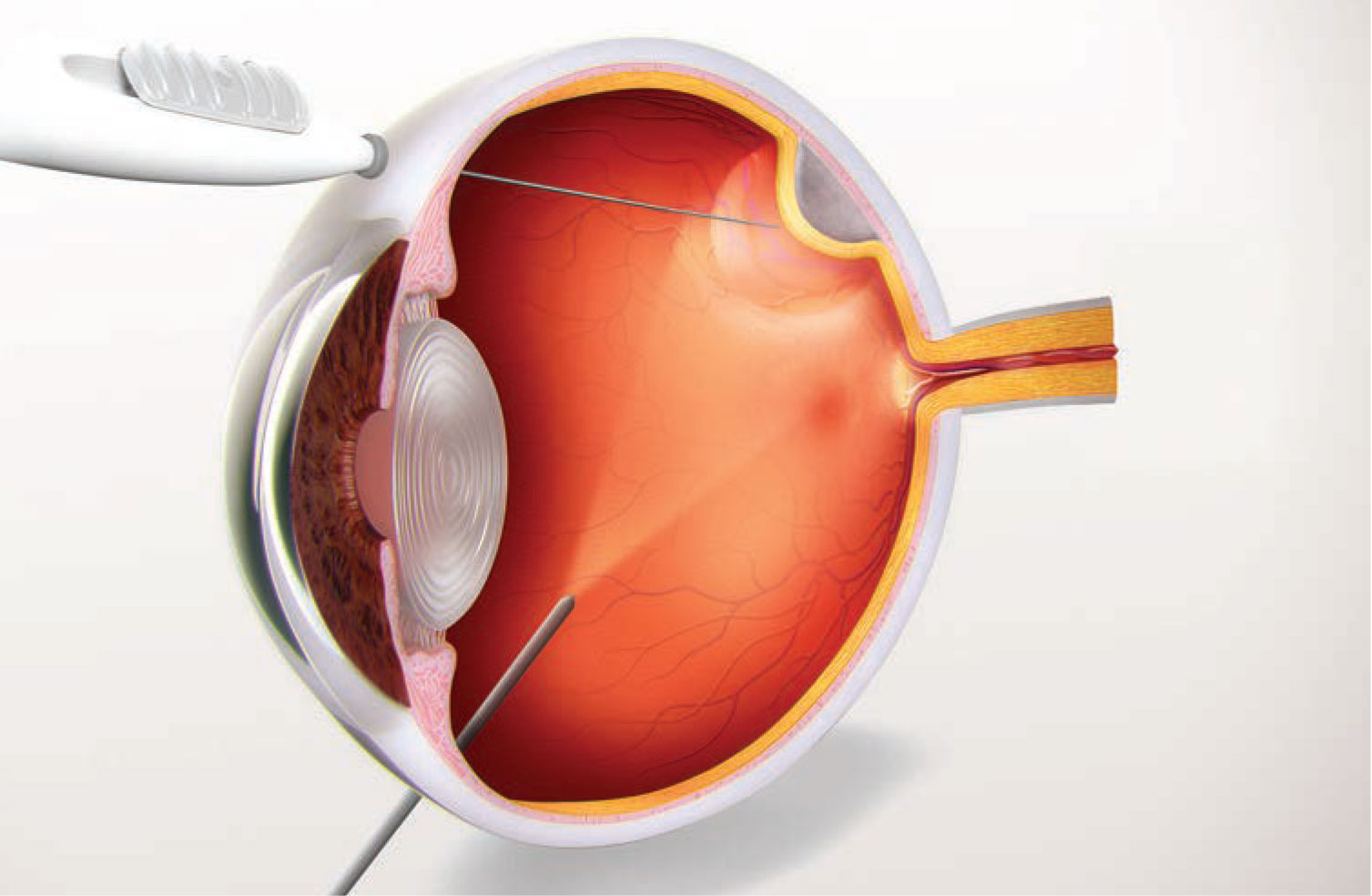 |
|
Figure 3. RGX-314, delivered as a onetime subretinal injection, as demonstrated in this illustration, is a recombinant adeno-associated virus gene therapy vector carrying a coding sequence for an anti-VEGF protein. |
A Phase I/IIa, open-label, multiple-cohort, dose-escalation study is under way and is expected to conclude in this summer (NCT04049266). The primary endpoint is safety at 26 weeks. Secondary endpoints, assessed at 106 weeks, include change in BCVA, change in central retinal thickness, mean number of rescue injections and mean change in area of choroidal neovascularization.7
Early data released by the company show treatment effect at three years, with four of six patients being injection-free from nine months to three years, and three of six patients being injection-free over three years.8 ATMOSPHERE, a Phase IIb/III clinical study, will enroll 300 patients and evaluate two doses of RGX-314 with a primary endpoint of mean BCVA relative to ranibizumab (NCT04704921). The estimated study completion date is March 2024.
Bottom line
Anti-VEGF therapy is extremely effective at maintaining and, in some cases, improving BCVA in patients with nAMD. However, the burden of treatment means that real-world outcomes often fail to mirror clinical trial results. So there’s a significant need to develop therapies that reduce the burden of frequent intravitreal injections. Several promising treatments in the pipeline may fundamentally change the treatment paradigm for nAMD as anti-VEGF therapies did and potentially provide better outcomes and improved quality of life for our patients. RS
REFERENCES
1. Rofagha S, Bhisitkul R, Boyer D, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON. Ophthalmology. 2013;120:2292-2299.
2. Campochiaro PA, Marcus DM, Awh CC, et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration results from the randomized Phase II Ladder clinical trial. Ophthalmology. 2019;126:1141-1154.
3. Compochiaro P. Primary analysis results of the Phase III Archway trial of the Port Delivery System with ranibizumab (PDS) for patients with neovascular AMD. Paper presented at the American Society of Retina Specialists virtual annual meeting. July 26, 2020.
4. Kim HM, Woo SJ. Ocular drug delivery to the retina: Current innovations and future perspectives. Pharmaceutics. 2021;13:108.
5. Ammar MJ, Hsu J, Chiang A, Ho AC, Regillo C. Age-related macular degeneration therapy: A review. Curr Opin Ophthalmol. 2021;31:215-221.
6. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, Phase III trial. Lancet. 2017;390:849-860.
7. Al-Khersan H, Hussain RM, Ciulla TA, Dugel PU. Innovative therapies for neovascular age-related macular degeneration. Expert Opin Pharmacother. 2019;20:1879-1891.
8. RegenxBio announces completion of dosing for Phase I/IIa clinical trial of RGX-314 in wet AMD. RegenXbio press release; May 30, 2019. http://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-completion-dosing-phase-iiia-clinical-trial. Accessed July 14, 2021.



