 |
 |
Despite the marked improvement anti-VEGF injections have meant in the prognosis of patients with exudative macular degeneration, many patients continue to have persistent disease activity regardless of frequent injections.
This raises a number of questions about predicting the effectiveness of anti-VEGF medications. Wouldn’t a marker that predicted anti-VEGF resistance be helpful in planning and following the care of patients with exudative macular degeneration?
What if an alternative treatment existed that actually resulted in better vision than anti-VEGF monotherapy at two years? Wouldn’t that be better than the approved and investigational anti-VEGF agents that allow for increased duration of anti-VEGF therapy but not necessarily better vision?
What if that same therapy required half the number of anti-VEGF injections over two years? That would be an improvement over the reported results of the existing anti-VEGF drugs.
What if this marker also showed that response was better with one specific anti-VEGF drug? Wouldn’t that be a huge help in determining which drug to start with in the management of exudative macular degeneration?
Pathophysiology of PCV
That marker may be polypoidal choroidal vasculopathy, also known as subretinal neovascularization with aneurysmal dilations. This subtype of exudative macular degeneration usually manifests as type I choroidal neovascularization between Bruch’s membrane and the retinal pigment epithelium. However, it can also have type II characteristics in which the CNV breaks through the RPE into the subretinal space.
Although PCV was initially theorized to be a choroidal vascular abnormality,1-2 optical coherence tomography studies have usually localized this lesion between Bruch’s membrane and the RPE.3-4 In the anatomic classification of J. Donald M. Gass, MD, for subretinal neovascularization,5 this would be a type I subretinal NV under the RPE and above Bruch’s membrane. Type II subretinal NV occurs above the RPE and in the subretinal space.5 Type III NV, or retinal angiomatous proliferation (RAP), includes an intraretinal component.5
The clinical presenting features of this aneurysmal form of CNV look very similar to what we see with exudative age-related macular degeneration: subretinal fluid and blood, as well as associated subretinal exudate and retinal pigment epithelial detachment (RPED).
PCV has some distinguishing clinical features compared to exudative AMD:6
- more subretinal fluid;
- higher height of subretinal fluid;
- more RPED;
- less intraretinal fluid or macular edema; and
- higher frequency of subretinal hemorrhage.
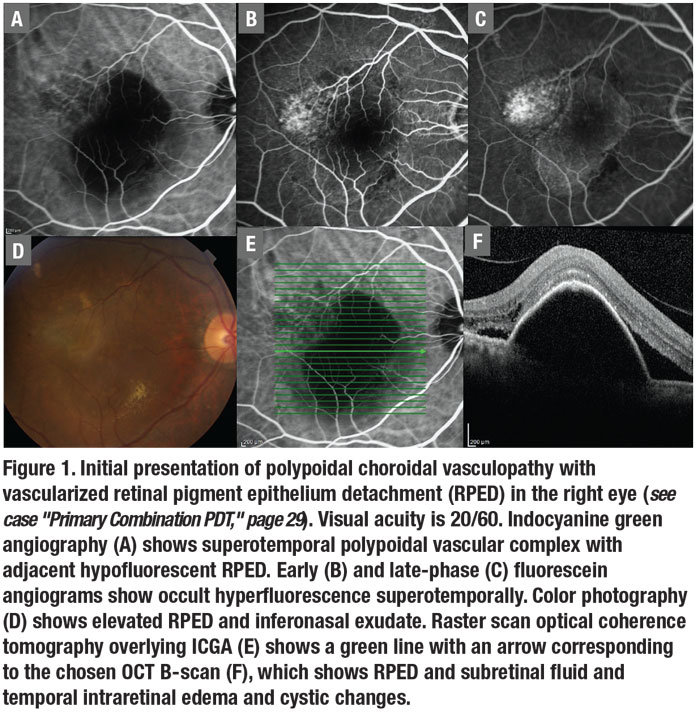
However, unlike with typical exudative AMD, the PCV diagnosis can’t be purely based on fundus examination or fluorescein angiography.7 FA in PCV for most cases shows occult leakage or occult CNV often associated with RPED (Figure 1 B, C).
 |
1. Isn’t PCV mainly a disease among Asians?
PCV has been diagnosed with high prevalence in Asian populations. However, PCV in Caucasians has been underdiagnosed due to a lack of access to, or interest in, indocyanine green angiography. Initial studies reported the prevalence of PCV in Caucasians to be less than 10 percent,8-9 but these studies were done with digital fundus camera ICGA, which is much less sensitive than the scanning laser ophthalmoscope (SLO) ICGA.10 SLO ICGA in Caucasian groups showed the prevalence of PCV ranged from 20 percent in a Duke study11 to 24.5 percent in a study of a patient population with predominantly European ancestry from Brazil,12 and as high as 31 percent in a Caucasian population in Hawaii.13
ICGA is the best way to diagnose PCV due to its ability to delineate the aneurysmal lesions in the CNV complex with the highest sensitivity. The aneurysmal lesions are best seen three to five minutes after ICG dye injection. A hypofluorescent ring often surrounds them.
Video ICGA with the SLO may show the infrequent but dramatic finding of pulsations of the polypoidal lesions diagnostic of PCV. RPED is a frequent finding associated with PCV (Figure 1 A, D–F). The RPED may mask the aneurysmal lesions, especially with the usual hypofluorescence noted in the area of the RPED on SLO ICGA (Figure 1 E, F). PCV lesions often appear at the edge of or at a notch in the RPED.
A frequent finding is a branching vascular network (BVN) connected to the polypoidal lesions. SLO ICGA is the most sensitive imaging modality for visualizing polypoidal lesions and the BVN, which makes it more suitable for diagnosing PCV than flash fundus camera ICGA.10
 |
2. Why is PCV important to diagnose? Doesn’t it respond the same way as exudative AMD to our standard-of-care treatments?
No genetic markers now exist for anti-VEGF resistance. However, one phenotypic marker is predictive of anti-VEGF resistance: subretinal aneurysmal lesions in the CNV complex, or PCV. While case studies first identified this,14,15 subsequent studies have confirmed a significantly higher rate of persistent disease activity in eyes with PCV when treated with the currently available anti-VEGF agents.
This was seen in a study of pro re nata ranibizumab (Lucentis, Genentech/Roche) treatment in Switzerland16 as well as a retrospective U.S. study that defined anti-VEGF resistance as lack of clinical response after four consecutive injections and showed a statistically significant higher prevalence of anti-VEGF resistance associated with PCV in both Asian and Caucasian patients.13 In fact, PCV was an even stronger predictor of anti-VEGF resistance in Caucasian vs. Asian patients. Thus, polypoidal or subretinal aneurysmal lesions associated with subretinal neovascularization is the one phenotypic marker predictive of anti-VEGF resistance.
3. Is there another treatment option for eyes resistant to anti-VEGF agents?
Because PCV may not respond to anti-VEGF medications, alternative treatments may need to be considered, especially if a patient exhibits a poor response to therapy. Recently published two-year results of the EVEREST II trial showed that primary treatment of combination photodynamic therapy (PDT) with anti-VEGF injection was superior to anti-VEGF monotherapy alone both in terms of anatomic response with closure of polypoidal lesions and visual improvement.17-19 In addition, at two years treatment burden was half of that of anti-VEGF monotherapy (12 vs. six injections).
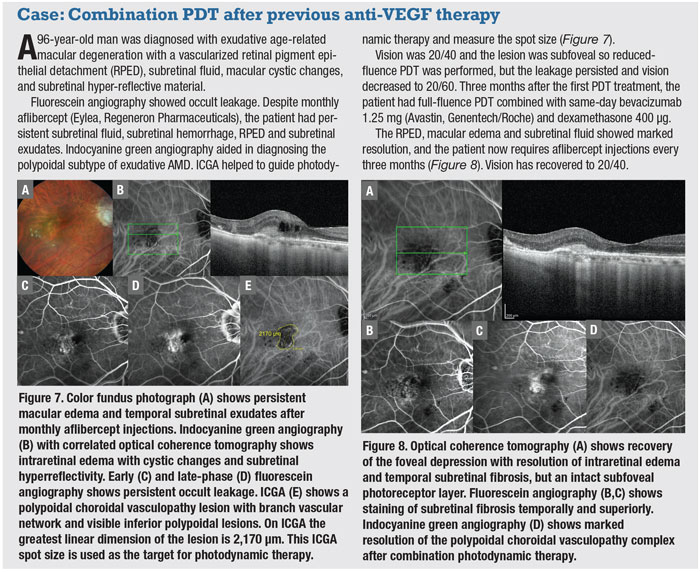
In addition to primary combination therapy of PDT and anti-VEGF therapy, eyes treated initially with anti-VEGF but with a poor response may have a better outcome with less treatment burden when subsequently treated with combination PDT.19 (See case examples on pages 29 and 30.)
4. How do we best diagnose the polypoidal or aneurysmal lesions within subretinal NV?
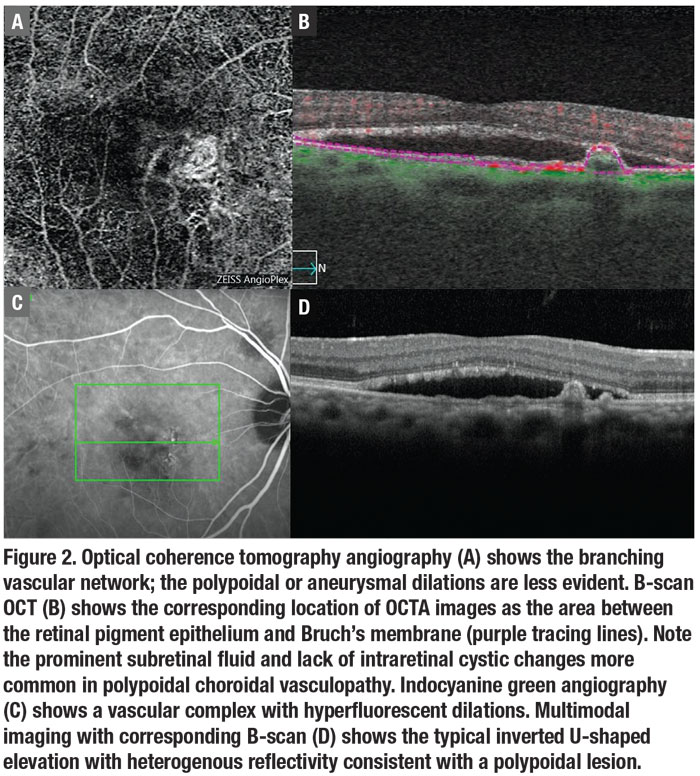
ICGA has been the gold standard for diagnosing polypoidal subretinal aneurysmal lesions in the subretinal neovascular complex,7,10,18-20 and SLO ICGA has greater sensitivity than digital fundus camera ICGA.10 However, if ICGA isn’t available, the diagnosis can be made using an imaging modality that has high specificity but lower sensitivity than ICGA. OCTA images the BVN well, although aneurysmal or polypoidal lesions are less discerning due to slower blood flow within the polypoidal lesions (Figure 2).21-22
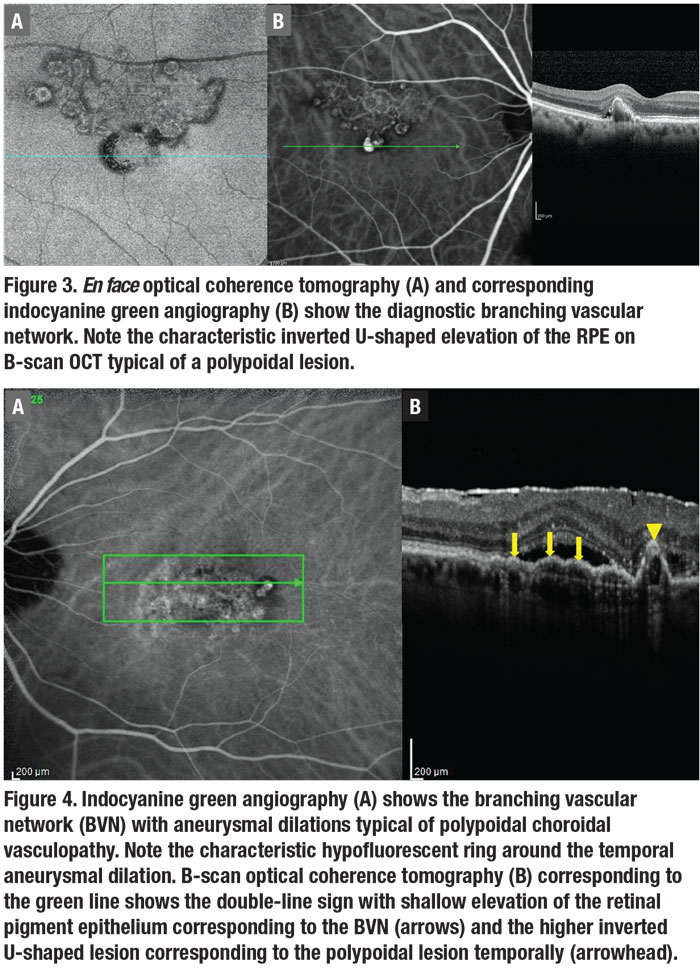
En face OCT can often demonstrate the BVN and the aneurysmal dilations
associated with PCV anatomically (Figure 3).21,23-24 But, again, it’s less sensitive than ICGA. B-scan OCT is the most available diagnostic modality in most practices, and shows fluid and blood associated with the polypoidal lesions.21-22 The polypoidal lesions most diagnostic of PCV appear as inverted U-shaped lesions with heterogeneous reflectivity, while the BVN appears as a shallow elevation of the RPE above Bruch’s membrane (double-line sign).4
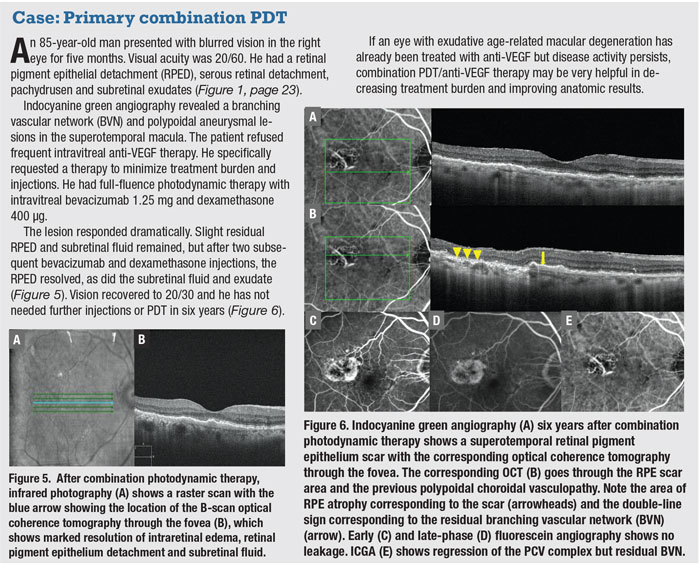
Practically, we recommend using any diagnostic means available to identify subretinal aneurysmal lesions, especially if the patient responds poorly to anti-VEGF therapy. Start with OCT B-scan, and don’t look just at the change images or the OCT map. Look for a double-line sign at the individual horizontal and vertical scans for areas suspicious for the BVN often with overlying fluid (Figure 4). Then look for polypoidal lesions and an inverted U-shaped elevation with heterogeneous reflectivity with B-scan OCT going through the lesions (Figures 2 to 4). These findings can resolve after anti-VEGF therapy, so it’s important to look at the B-scan OCT images before beginning anti-VEGF therapy.
The best way to fully evaluate an area with OCT is to perform the sequential raster scan, which allows you to scroll down through the entire macular with B-scan OCT. The en face mode is available on most OCT platforms and can be utilized from preexisting OCT data to scan a layer between the RPE and Bruch’s membrane, or between the outer retina and the choriocapillaris (ORCC). This may allow imaging of the BVN with the diagnostic polypoidal lesions or aneurysmal dilations.
OCTA localized to the area between the RPE and Bruch’s membrane or the ORCC cut may provide diagnostic pictures (Figure 2), but the polypoidal lesions tend to have low flow and may not visualize well with OCTA.19 However, multimodal imaging may identify the polypoidal lesions on B-scan when combined with en face OCT or OCTA (Figure 2).
 |
5. How does PCV treatment differ from that for wet AMD?
Based on the EVEREST II study, a reasonable approach is anti-VEGF therapy combined with PDT for PCV that involves the central fovea.17-19 Practically, if vision is still very good (20/40 to 20/50 or better), anti-VEGF therapy is a reasonable approach to start. However, if vision is 20/50 to 20/60 or worse, combination PDT and anti-VEGF therapy initially is a reasonable approach. In EVEREST II, combination PDT/anti-VEGF showed better results for treatment burden and visual recovery.
EVEREST II had no cases of sudden vision loss after full-fluence PDT. The trial did report a potential risk of choroidal ischemia or subretinal hemorrhage after PDT treatment, but this risk is small and is probably less than it is in typical exudative AMD due to the associated thick choroid. Especially if lesions are extrafoveal and the PDT lesion spot can avoid the fovea, combination PDT/anti-VEGF with the laser spot size sparing the fovea is a reasonable approach.
6. Does PCV respond as well to anti-VEGF as typical exudative AMD?
The response of typical exudative AMD to anti-VEGF therapy has been significantly better than the natural course of the disease, resulting in markedly less subretinal hemorrhage and leakage as well as better overall vision outcomes. However, case series and retrospective studies have shown the PCV subtype has a higher risk of anti-VEGF resistance.13-16 PCV eyes on anti-VEGF therapy have more persistent disease activity, again making the subretinal aneurysmal lesions diagnostic of PCV as the only phenotypic marker for anti-VEGF resistance in eyes presenting with exudative AMD.
 |
7. Is combination PDT/anti-VEGF for PCV different from that for typical exudative AMD?
In the early 2000s, FA was used to determine lesion size in typical wet AMD and the area of leakage was delineated. The greatest linear dimension was calculated based on leakage on FA. The initial recommendation was to use a treatment spot 1,000 µm larger than the greatest linear dimension leakage on the FA for typical exudative AMD. This involved a large area, including significant adjacent normal RPE.
PDT treatment for PCV lesions as performed in the EVEREST II study is very different. The PCV spot size for PDT is based on ICGA, not FA. The area of BVN and the polypoidal lesions is encircled. The greatest linear dimension is then determined on ICGA. Some experts use a treatment spot size exactly the size of the PCV lesion on ICGA. However, it’s also reasonable to add a 300-µm border around the lesion on ICGA.
For verteporfin (Visudyne, Bausch + Lomb), an intravenous dose of 6 mg/m2 is given and the diode laser (689 nm) is directed to the treatment area 15 minutes after intravenous dye infusion. If the PCV lesion is extrafoveal, we recommend full-fluence PDT (50 J/cm2 of light at 600 mW/cm2 for 83 seconds). If the lesion is subfoveal and vision is good, then reduced- fluence PDT (25 J/cm2 at 300 mW/cm2) can be considered.
Laser-spot duration is 83 seconds for both full-fluence and reduced-fluence treatment, but the laser settings are different as noted previously. If vision is 20/50 to 20/60 or worse, full-fluence treatment is reasonable for subfoveal lesions based on the EVEREST II study.
 |
8. Does macular laser photocoagulation of polypoidal lesions have a role in PCV therapy?
If the diagnosis of PCV is made with extrafoveal polypoidal lesions resulting in leakage, focal macular laser treatment to the polypoidal lesions is reasonable with or without supplemental anti-VEGF therapy. This may stabilize the leakage long-term with good vision. The goal of thermal laser is to close the polypoidal lesion and prevent further leakage or bleeding.25
9. Among ethnic populations, do the presenting characteristics of PCV differ?
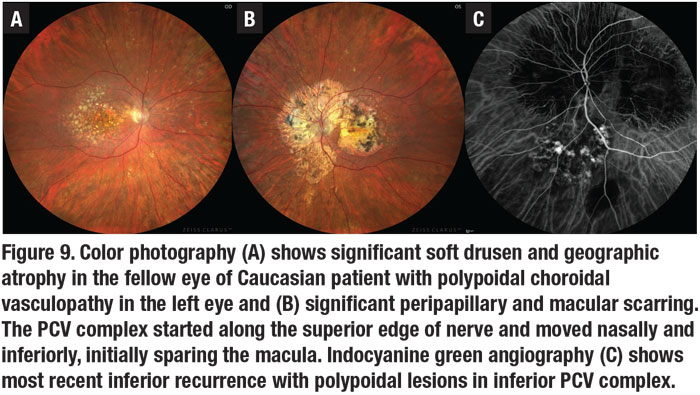
PCV was initially described as a peripapillary disease in Caucasian and Black patients and often bilateral.9 More recent studies in Caucasians showed that PCV primarily affects the macula.26 In Caucasians with unilateral disease, significant drusen and geographic atrophy can present in the fellow eye (Figure 9A), and peripapillary disease provides a characteristic peripapillary scarring around the nerve (Figure 9B), which doesn’t usually occur in Asian patients with this disease.
Although typical exudative AMD has long been known to be more frequent in females, PCV has a strong male predilection in Asians vs. the usual female predominance of typical exudative AMD in Caucasians.13,26 PCV in Blacks often has larger caliber vessels and is more often peripapillary.9
 |
10. So what role does diagnosing PCV in exudative AMD patients have in my practice?
Although most patients with exudative AMD receive anti-VEGF as first-line therapy, PCV is the one subtype of exudative AMD that may predict anti-VEGF resistance.
If a patient with exudative AMD has a poor response to first-line anti-VEGF therapy, alternative treatment with combination PDT/anti-VEGF injection can be considered. However, if only B-scan OCT is available, polypoidal or aneurysmal lesions may regress after long-term anti-VEGF therapy, making the diagnosis of PCV more difficult.
In addition, EVEREST II has shown that combination therapy with PDT should be considered as a primary treatment for PCV because it yields better vision and anatomical results than ranibizumab alone.17-18 Finally, PCV may be more responsive to certain anti-VEGF medications. Aflibercept (Eylea, Regeneron Pharmaceuticals) is the treatment of choice in Asia for PCV; it has shown a significantly better response in some eyes treated previously with other anti-VEGF agents27 and, in the PEARL II trial, even in patients previously treated with high-dose ranibizumab.4
In the future, there could also be treatment response differences with newer medications, such as brolucizumab (Beovu, Novartis), based on treatment responses in the HAWK and HARRIER trials, as well as anticipated results from the ongoing Merlin trial for previously treated anti-VEGF-resistant exudative AMD.
Bottom line
PCV is the most impactful subtype of exudative AMD because it provides a marker for anti-VEGF-resistance, which may affect therapeutic planning for treatment-naïve eyes as well as eyes responding poorly to anti-VEGF therapy. Combination PDT/anti-VEGF therapy can significantly decrease treatment burden and improve anatomic and visual outcomes.
Although SLO ICGA is the gold standard for diagnosing PCV, retina specialists should use all modalities, including B-scan OCT, en face OCT and OCTA, to make the diagnosis. While common in Asian patients, PCV is more common in Caucasian patients than previously thought (20 to 30 percent of exudative AMD). RS
REFERENCES
1. Yannuzzi LA. Idiopathic choroidal vasculopathy. Paper presented at Macula Society annual meeting; February 5, 1982; Miami, FL.
2. Yannuzzi LA, Sorensen J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy. Retina. 1990;10:1–8.
3. Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy. Simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012; 32:1057–1068.
4. Kokame GT. Prospective evaluation of subretinal location in polypoidal choroidal vasculopathy (PCV) and response of exudative and hemorrhagic PCV to high dose anti-angiogenic therapy (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2014;112:74–93.
5. Gass JDM. Stereoscopic atlas of macular diseases: Diagnosis and treatment. 4th ed. St. Louis: Mosby; 1997.
6. Ozawa S, Ishikawa K, Ito Y, et al. Differences in macular morphology between polypoidal choroidal vasculopathy and exudative age-related macular degeneration detectd by optical coherence tomography. Retina. 2000;29:793-802.
7. Kokame GT. Polypoidal choroidal vasculopathy—an important diagnosis to make with therapeutic implications. Retina 2012;32:1446–1448.
8. Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752-759.
9. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol. 1999;117:1503-1510.
10. Cheung CM, Lai TY, Chen SJ, et al. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: The group experience with digital fundus photography and scanning laser ophthalmoscopy. Retina. 2014; 34:2397-2406.
11. Mettu PS, Allingham J, Nicholas PC, Cousins SW. Neovascular morphology by ICG angiography and response to loading dose anti-VEGF therapy in patients with neovascular AMD. IOVS 2016; 57:2.
12. Pereira FB, Veloso CE, Kokame GT, Nehemy MB. Characteristics of neovascular age-related macular degeneration in Brazilian patients. Ophthalmologica. 2015;234:233-242.
13. Kokame GT, de Carlo TE, Kaneko KN, Omizo J, Lian R. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology Retina. 2019;3:744-752.
14. Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010;150:666-673.
15. Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148:70-81.
16. Hatz K, Prunte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98:188-194.
17. Kokame GT, Kim JE. Treatment for a subtype of exudative macular degeneration: Another mountain climbed. JAMA Ophthalmol. 2020;138:942-944.
18. Lim TH, Lai TYY, Takahashi K, et al. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. JAMA Ophthalmol. 2020:138:935-942.
19. Koh A, Lai TY, Takahashi K, et al. Efficacy and safety of ranibiuzmab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. A randomized clinical trial. JAMA Ophthalmol. 2017;135:1206-1213.
20. Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125:708-724
21. deCarlo T, Kokame GT, Kaneko KN, Lian R, Lai JC, Wee R. Sensitivity and specificity of detecting polypoidal choroidal vasculopathy with en face OCT and OCT angiography. Retina. 2019; 39:1343-1352.
22. deCarlo T, Kokame GT, Shantha JG, et al. Spectral domain optical coherence tomography angiography for the diagnosis and evaluation of polypoidal choroidal vasculopathy. Ophthalmologica. 2018;239:103-109.
23. Kokame GT, Hirai K, Yanagihara R. Polypoidal choroidal vasculopathy: Imaging by indocyanine green angiography and en face optical coherence tomography. JAMA Ophthalmol. 2015;133:e151886.
24. Sayahagi K, Gomi F, Akiba M, et al. En-face high penetration optical coherence tomography imaging in polypoidal choroidal vasculopathy. Br J Ophthalmol 2015;99:29-35.
25. Kokame GT. Polypoidal choroidal vasculopathy – A type I polypoidal subretinal neovasculopathy. Open Ophthalmol J. 2013; 7:82-84.
26. Kokame GT, Liu K, Kokame KA, Kaneko KN, Omizo JN. Clinical characteristics of polypoidal choroidal vasculopathy and anti-vascular endothelial growth factor treatment response in caucasians. Ophthalmologica 2020;243:178-186.
27. Kokame GT, Lai JC, Wee R, et al. Prospective clinical trial of intravitreal aflibercept treatment for polyploidal choroidal vasculopathy with hemorrhage or exudation (EPIC study): 6 month results. BMC Ophthalmology. 2016;16:1-10.



