She reported the first treatment was not effective, but the second treatment led to a modest reduction in the subretinal fluid. She was subsequently treated with verteporfin photodynamic therapy (PDT) without complete resolution of the fluid in the left eye. The patient noted increased stress recently from moving to Seattle and starting a new job one month before her visit. She works as a physician-scientist researcher.
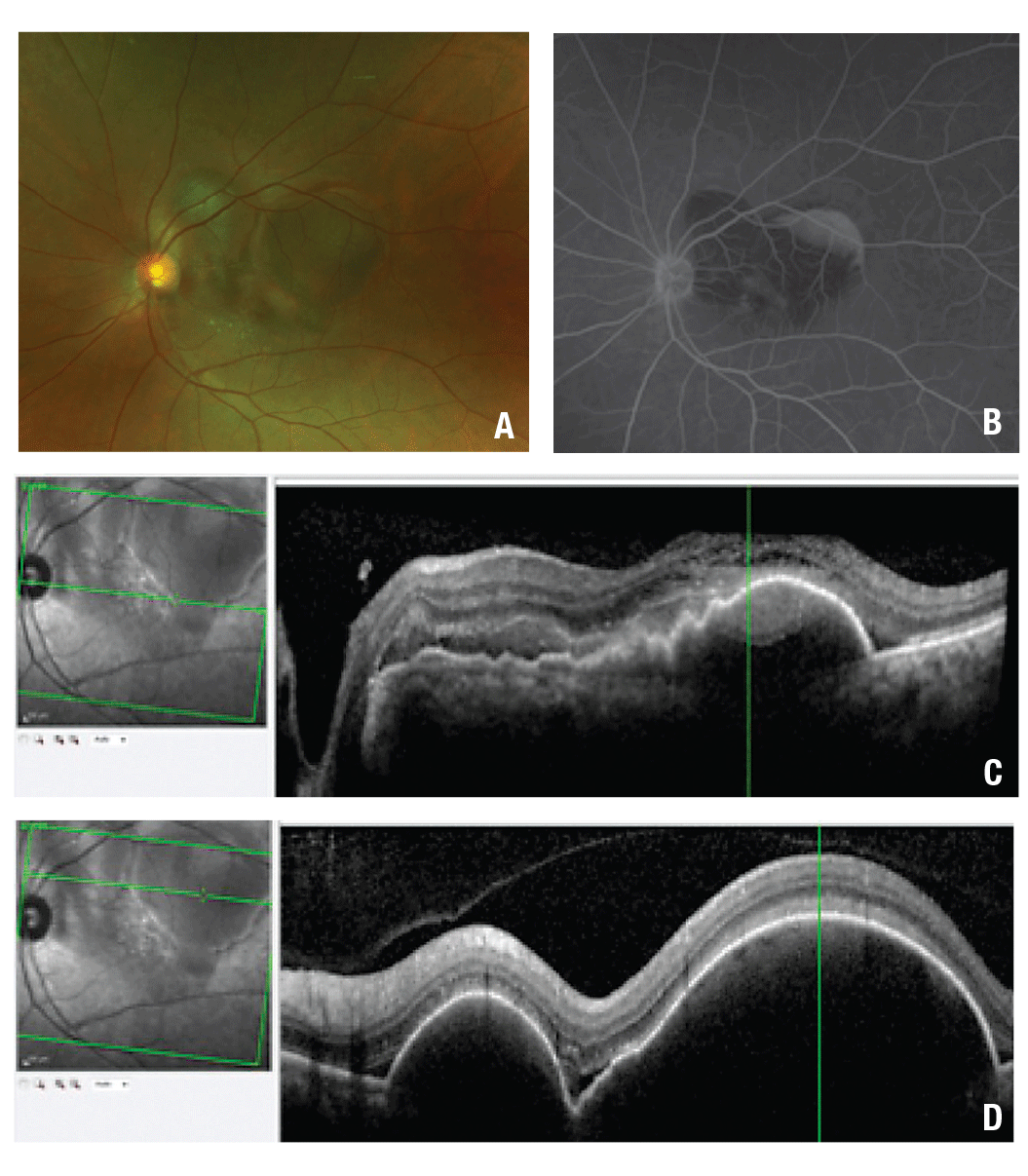 |
| Figure 1. Color fundus photography at presentation (A) shows multiple pigment epithelial detachments (PED) associated with subretinal hemorrhage. The initial fluorescein angiogram of the left eye (B) shows blockage in the macula from the subretinal hemorrhage, late pooling within the PED in the temporal macula and diffuse and pinpoint leakage in the central macula. Optical coherence tomography (C) shows a central macular PED with adjacent subretinal hemorrhage. Raster scans through the superior macula (D) show large PEDs and small pockets of subretinal fluid. |
Examination Findings
Best-corrected visual acuity was 20/20 and 20/40 in the right and left eyes, respectively. Intraocular pressures were normal, as were pupils, extraocular movements and confrontational visual fields. The anterior segment examination was only notable for trace nuclear sclerosis cataracts in both eyes.
The dilated fundus examination of the right eye showed a posterior vitreous detachment and a mild epiretinal membrane. In the left eye, the dilated fundus exam was notable for large-diameter, highly elevated pigment epithelial detachments (PEDs) with adjacent subretinal hemorrhage (SRH) concentrated in the superonasal macula (Figure 1A). No drusen, geographic atrophy, or pigment mottling were present in either eye.
Ancillary Testing
Fluorescein angiography with transit in the left eye showed normal transit time and vascular filling. However, there was blockage throughout the macula due to the subretinal hemorrhage. Late pooling was noted superiorly within the most temporal part of the PED. Diffuse and pinpoint leakage was also observed in the macula (Figure 1B). Optical coherence tomography of the left eye showed large serous PEDs with shallow surrounding SRH in the macula and along the superior arcade. Subretinal fluid (SRF) was also noted (Figure 1C).
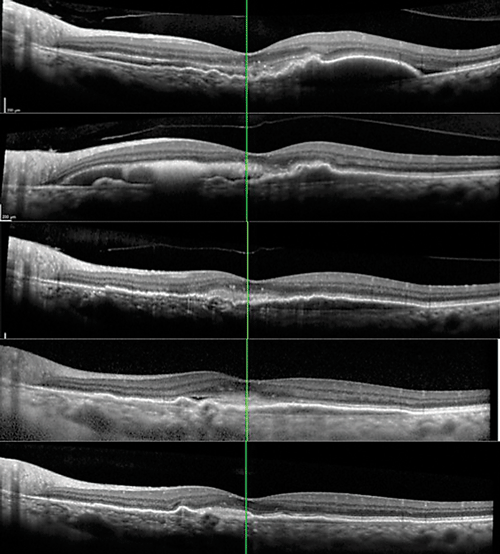 |
| Figure 2. Progression of optical coherence tomography raster scans through the fovea at (A) presentation before treatment in our clinic; (B) one month after presentation and after one injection of bevacizumab; (C) four months after presentation, after three bevacizumab and one aflibercept injections; and (D) 12 months after the initial visit, after three bevacizumab and fi veaflobercept injections and one photodynamic therapy session. Thirteen months after presentation and an additional aflibercept injection, the most recent image (E) showed flattening of the shallow pigment epithelial detachment central macula, and resolution of the subretinal fluid. |
The patient’s acute hemorrhage was suspicious for a choroidal neovascular membrane (CNVM). Additionally, the leakage on FA was suggestive of CNVM vs. chronic CSCR. The differential diagnosis included wet age-related macular degeneration, polypoidalchoroidal vasculopathy (PCV) or CSCR with secondary CNVM.
We treated the hemorrhage and macular fluid with additional intravitreal bevacizumab injections. One month after the first injection, the subretinal hemorrhage displaced inferiorly just nasal to the fovea. She had three more monthly bevacizumab injections with near resolution of the subretinal hemorrhage, then we switched to intravitreal aflibercept (Eylea, Regeneron). With ongoing treatments, the patient achieved excellent anatomic recovery with flattening of the macular PED and stable visual acuity (Figure 2A to D).
After her third monthly aflibercept injection, the patient was lost to follow-up for seven weeks. She returned with enlargement of the PED in the central macula and an enlarged hemorrhagic PED along the superior arcade of the left eye (Figure 3A). Her visual acuity had decreased to 20/50.
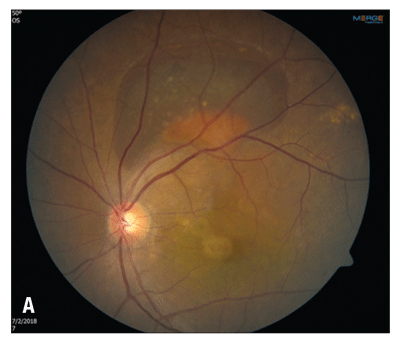  |
| Figure 3. Fluorescein angiography with transit of the left eye shows dilated, telangiectatic and aneurismal vessels temporal to the foveal center with slow petalloid leakage around and temporal to the fovea in late frames. |
At this point, we restarted aflibercept on a monthly basis, resulting in improvement of the macular PED, but with persistence of the PED along the superior arcade. We also prescribed oral eplerenone up to 50 mg daily for four months, but ultimately discontinued the medication because it did not appear to be helpful.
One month later, we treated the PED along the superior arcade with PDT followed by an aflibercept injection. The combination resulted in flattening of the shallow PED in the central macula (Figure 2D). Her visual acuity recovered to 20/30 in the left eye. The PED along the superior arcade persisted, but ultimately decreased in size
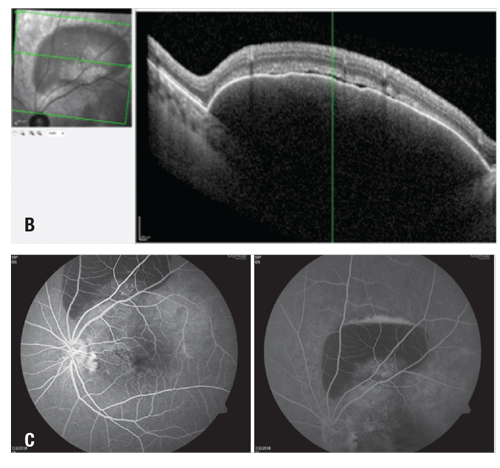
The patient had OCT angiography approximately one year into treatment so we could characterize the choroidal neovascular membrane. The right eye appeared normal, but the left eye showed two large branching networks of neovascular vessels in the sub-RPE space (Figure 4, page 17). One focus was located in the central macula and another at the inferior border of the PED along the superior arcade.
 |
| Figure 4. Optical coherence tomography angiography (from the outer retina to the choriocapillaris layer) reveals extensive vascular networks in the subretinal pigment epithelium space and within the pigment epithelial detachments of the left eye |
Given this patient’s demographics, imaging characteristics and response to the combination of anti-VEGF and PDT, our leading diagnosis is PCV. Debate remains whether PCV represents a subtype of neovascular AMD or a completely separate entity. Both diseases share similarities, such as the presence of CNVM and associated predisposing genes such as HTRA1 (HtrA Serine Peptidase 1) and variants in CETP (cholesteryl ester transfer protein).1
Patients with PCV are mostly Asian or African-American. Many cases of wet AMD manifest with features of PCV in these populations. PCV occurs in Caucasians less frequently than wet AMD.2 Studies suggest that 54.7 percent of Japanese patients with neovascular AMD also have PCV, in contrast to 9.8 percent of Caucasians.3 PCV patients are also younger than AMD patients—usually age 50 to 65 years. PCV is more often unilateral in Asians and bilateral in Caucasians.1
The etiology of PCV is poorly understood. However, it is characterized by serosanguinous detachments of the pigmented epithelium, exudation and subretinal fluid. Other findings include subretinal fi brosis and atypical branching vascular networks with terminal aneurysmal dilatations referred to as polyps, often localized to the peripapillary region.4 In PCV, drusen are few and rare, or even absent; in AMD, drusen are the characteristic sign.1 Although PCV has been associated with multiple recurrent serous retinal detachments, it results in much less fibrous proliferation compared to end-stage neovascular AMD.5
How to Differentiate PCV
Although AMD and PCV may be on the same spectrum of disease, it is important to differentiate them because demographics, natural history, visual prognosis and management differ significantly.6 On FA, PCV lesions resemble occult CNVM lesions and can be mistaken for AMD when localized in the central macula.
Indocyanine green angiography, with its ability to highlight choroidal vasculature, is the standard for diagnosis of polypoidal lesions. The polyps present as focal hyperfluorescent spots. In the later stages of the disease, the dye pattern reverses as the center of the lesion becomes hypofluorescent with surrounding hyperfluorescence.6
OCT can also show double reflective layers that consist of retinal pigment epithelium and another highly reflective layer beneath the RPE—known as “double-layer sign”— in the area of the branching network vessels. OCT can also show a notch in the margin of large PED, indicating the site of polypoidal lesions.7
Several studies have evaluated the treatment of PCV. The efficacy of anti-VEGF agents is well known for CNV secondary to AMD.8 However, some authors have postulated that the pathophysiology of PCV is less driven by vascular endothelial growth factor than wet AMD. Studies have substantiated this claim, showing significantly lower aqueous VEGF levels in PCV compared to wet AMD.9 Although anti-VEGF injections do show efficacy, the underlying choroidal abnormalities in PCV have been shown to be more responsive when PDT is employed.10
Combination Therapy
The combination therapy of PDT and an anti-VEGF agent has shown superior results, including improved vision, reduced SRH and reduced recurrence of polyps compared to either alone. The EVEREST trial compared PDT combined with ranibizumab vs. monotherapy of either treatment in patients with macular PCV.11 The combination arm achieved the best visual and angiographic outcome, with the highest proportion of complete polypoidal lesion regression (78 percent) and highest mean gain in vision (+10.9 letters).
Recently, the PLANET trial compared intravitreal aflibercept monotherapy to aflibercept with adjunctive PDT in 318 older adults with symptomatic PCV. The study showed aflibercept monotherapy was noninferior to aflibercept plus PDT. However, the study could not elucidate the benefits of adding PDT because most participants responded to intravitreal aflibercept alone.12
In summary, PCV can occur in any sex or race. It is more commonly seen in the peripapillary area, without associated drusen, and in nonwhite patients. PCV is diagnosed with ICGA showing leakage from a vascular network of polyps and visible exudation associated with PED. OCTA may allow excellent visualization of these structures both for diagnosis and to assess therapeutic response. Lastly, evidence shows that PDT may serve as an effective adjunct to traditional anti-VEGF medications used for wet AMD, but that aflibercept monotherapy may also be an effective approach.

References
1. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19-29.
2. Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA. Polypoidal choroidal vasculopathy Surv. Ophthalmol. 2009;49:25-37.
3. Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15-22.
4. Kokame GT, Yeung L, Lai J. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br. J. Ophthalmol. 2010;94:297-301.
5. Jager RD, Mieler WF, Mille JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606-2617.
6. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115:478-485.
7. Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27:589-94.
8. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363-372 e365.
9. Tong JP, Chan WM, Liu DT et al. Aqueous humor levels of vascular endothelial growth factor and pigment epitheliumderived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456-462.
10. Gomi F, Sawa M, Wakabayashi T et al. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2010; 150:48-54 e41.
11. Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH, for the EVEREST Study Group. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomized controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:624-8.
12. Lee W, Lida T, Ogura Y et a. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study. A randomized clinical trial. JAMA Ophthalmol. 2018;136:786-793.



