Take-home Points
|
 |
|
Bios Dr. Nguyen is an ophthalmology resident at the University of Texas Health Science Center in San Antonio. Dr. Singer is clinical professor of ophthalmology at the University of Texas Health Science Center in San Antonio and director of clinical research at Medical Center Ophthalmology. DISCLOSURES: Dr. Nguyen has no relevant financial relationships to disclose. Dr. Singer disclosed relationships with Aerie Pharmaceuticals, Allegro Ophthalmics, Allergan/AbbVie, EyePoint Pharmaceuticals, Genentech/Roche, Kodiak Sciences, Mallinckrodt, Novartis, Regeneron Pharmaceuticals, Santen Pharmaceutical, Spark Therapeutics, Icon, Ionis Pharmaceuticals, Kalvista, Opthea, Optos, Senju and Sydnexis. |
As we all know, anti-VEGF medications have transformed how we care for multiple retinal pathologies. However, the treatment schedule for each anti-VEGF medication varies based on its duration of action, disease activity and treat-and-extend schedule with individual patients, especially for neovascular age-related macular degeneration. The finite window of efficacy per injection creates a revolving door of injections for each affected eye. And refractory cases of diabetic macular edema and nAMD may accelerate the revolving door with the need for trial-and-error of various anti-VEGF agents, with or without adjunctive ocular steroids.
The marketplace has responded to these clear demands with progressively more potent anti-VEGF candidates with longer durations of action. However, brolucizumab (Beovu, Novartis) has been heavily scrutinized due to its higher reported incidence of mild to severe intraocular inflammation despite its potent “drying” effects and longer duration.
While brolucizumab finds its niche in the treatment arsenal in addressing refractory cases of nAMD, the question remains: What patients are at highest risk of suffering visually significant IOI? Answering this question can help to triage appropriate candidates and guide development of potentially safer intravitreal anti-VEGF formulations. We tackle that question here.
Early reports of IOI
IOI has been marked as an adverse event of interest since the VIEW study, which identified an incidence of 0.005 to 1.5 percent in patients receiving intravitreal ranibizumab (Lucentis, Genentech/Roche) and 0.5 to 1.1 percent with intravitreal aflibercept (Eylea, Regeneron Pharmaceuticals).1 The higher incidence of IOI in 6-mg brolucizumab (4.6 percent in HAWK and HARRIER), and potentially more severe sequelae leading to vasculitis (3.3 percent) and occlusive vasculitis with retinal vascular occlusion (2.1 percent) and irreversible vision loss is unique to the agent (Figures 1 and 2).2
Two groups have examined this adverse effect in depth since brolucizumab launched in October 2019: the Research and Safety in Therapeutics (ReST) panel established by the American Society of Retina Specialists; and Novartis’ own safety review committee, an independent group of experts charged to investigate reported cases of IOI in HAWK and HARRIER. The two groups were tasked to determine any trends that might elucidate the underlying cause.
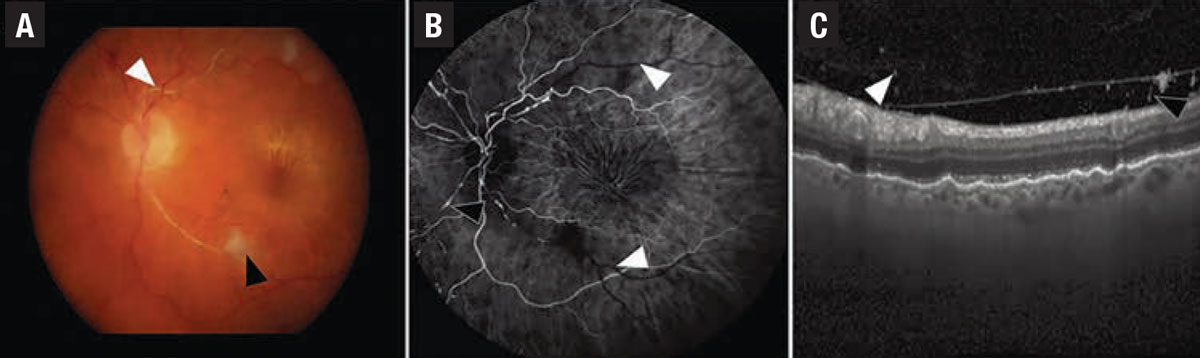 |
| Figure 1. In a case of iridocyclitis and retinal arterial thrombosis from HAWK and HARRIER, A) color fundus photography demonstrates whitening of the retinal artery consistent with retinal artery occlusion (white arrowhead) and a cotton wool spot (black arrowhead). B) Fluorescein angiogram in the venous phase demonstrates nonperfusion of the retinal arteries (white arrowheads) and arterial box-carring (black arrowhead). C) Spectral-domain optical coherence tomography demonstrates cells in the vitreous on the posterior hyaloid. (Source: Singer M, et al. Ophthalmol Retina. Published online May 8, 2021: doi: 10/1016/j.oret.2021.05.003.) |
The two groups’ conclusions are congruent and ultimately recommend use of brolucizumab only in patients who have had a careful examination to rule out active intraocular inflammation.1,2 We compared postmarket data with HAWK and HARRIER post-hoc analyses to contextualize potential etiologies and risk factors for IOI after anti-VEGF injections.
Drilling down into HAWK and HARRIER
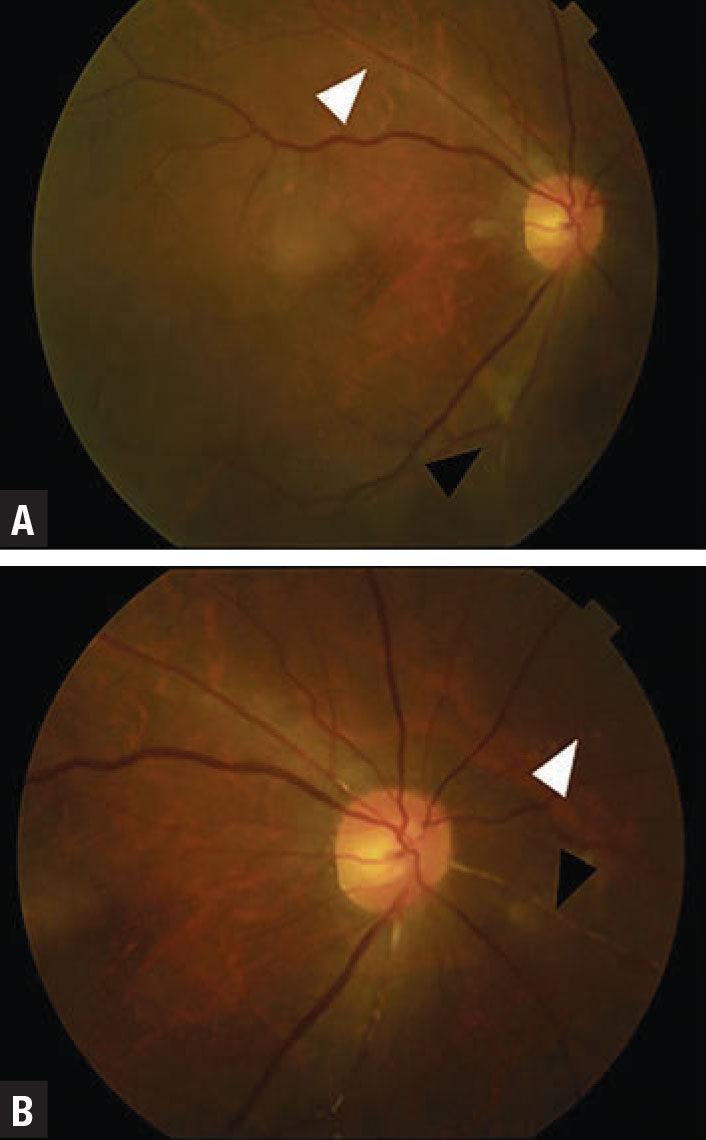 |
| Figure 2. A case of uveitis and retinal artery occlusion from HAWK and HARRIER. Color fundus photographs show small and focal narrowing of retinal arterioles (white arrowheads) and occlusion (black arrowheads). (Photo: Singer M, et al. Ophthalmol Retina. Published online May 8, 2021: doi: 10/1016/j.oret.2021.05.003.) |
HAWK and HARRIER were Phase III clinical trials designed to determine the efficacy of brolucizumab (3 mg and 6 mg in HAWK, 6 mg in HARRIER) in treating nAMD relative to aflibercept 2 mg.3 Enrolled patients were treatment-naïve and received baseline fluorescein angiography and spectral-domain optical coherence tomography before starting three monthly loading doses. Brolucizumab recipients were then evaluated and, if they qualified, maintained on q12-week dosing but changed to q8-week dosing if OCT and vision criteria showed evidence of disease activity.
Slightly more than half of the patients in the brolucizumab arms continued on the q12-week dosing within the first year. In terms of AEs, 49 of the 1,088 eyes treated with brolucizumab had at least one IOI AE at a mean time of occurrence at 100 days, and 18 days from the last injection.4 Eighty-seven percent were treated with topical steroids and others were observed or treated with oral or intraocular steroids.
Among the patients treated for IOI, 80 percent achieved resolution, 10 percent did so with sequelae and 10 didn’t show any resolution. Thirty-six eyes with one prior IOI event continued on brolucizumab, of which 12 suffered another IOI event and discontinued the study.
The unique AE of IOI and occlusive vasculitis occurred in 2.1 percent of eyes, of which five of seven eyes (71 percent) resulted in vision loss of >15 letters. The overall impact of IOI AEs on vision was a maximum loss of 16.31 EDTRS letters within three months of the culprit injection-, a loss of 0.22 letters when tracked to the end of study.2
 |
It’s interesting to note that among patients who developed IOI, more gained letters by the end of the study, but the vast majority who developed vasculitis and or vascular occlusion lost the most vision. The post-hoc analyses didn’t compare IOI between aflibercept and brolucizumab, but noted that aflibercept had less than a 1-percent incidence of IOI; all cases were mild or moderate in severity.
Postmarket data
The reported incidence of IOI in postmarket data from Novartis has fluctuated from greater than to less than that of HAWK and HARRIER. By March 2020, approximately 65,000 to 70,000 injections had been given to 37,000 eyes with vasculopathies noted in 26 eyes of 25 patients.4 The reported incidence of occlusive vasculitis as of August 2020 was 4.71 per 10,000 injections, usually in the presence of IOI.1
Although data are still evolving, the occlusive vasculopathy incidence per injection in HAWK/HARRIER is still three times the rate in postmarket data, which suggests underreporting. One reason could be the difficulty differentiating occlusive vasculopathy in the presence of IOI from postinjection endophthalmitis, which also presents at a rare incidence of 0.02 percent.5
 |
Smaller real-world studies for nAMD (non-treatment-naïve patients, n=152) have also reported incidences of elevated ranges of brolucizumab-induced inflammation at 8.1 percent for IOI, 0.6 percent for occlusive vasculitis, and 1.2 percent for severe vision decline in IOI eyes.6
The Komodo Health Database and IRIS registry have reported postmarket IOI incidence of 2.4 percent with vasculitis and occlusion incidence of 0.55 percent. Their multivariate regressions revealed a 4.5-percent risk of an occlusive event within the first six months of a prior IOI event (eight times baseline risk) and 10-percent risk of repeat IOI (four times baseline risk).7
MERLIN (NCT03710564, n=529) was a clinical trial that compared brolucizumab 6 mg q4-week to aflibercept 2 mg q4-week in previously treated patients that have persistent retinal fluid. Data as of July reported the incidence of IOI, vasculitis and occlusion in brolucizumab vs. aflibercept at 9.3 vs 4.5 percent, 0.8 vs 0 percent and 2 vs 0 percent. As a result the trial was halted.8
KITE (NCT03481660, n=361) and KESTREL (NCT 03481634, n=571), parallel studies targeting the DME population as HAWK and HARRIER did for those with nAMD, also compared brolucizumab 3 and 6 mg and aflibercept 2 mg (KESTREL) or brolucizumab 6 mg and aflibercept 2 mg (KITE). The brolucizumab arms had q6-week interval loading doses before continuing on a q8- to q12-week regimen. The spaced out loading doses for brolucizumab revealed IOI, vasculitis and occlusion rates vs. aflibercept (q4-week loading doses) of 1.7 to 4.7 percent vs. 0.5 to 1.7 percent, 0 to 1.6 percent vs. 0 percent, and 0.5 to 1.1 percent vs. 0.3 to 0.6 percent, respectively.9
| Risk factors for IOI and how to manage it Several clues hint at a delayed hypersensitivity event in cases of intraocular inflammation after injection of brolucizumab. HAWK/HARRIER showed the duration between prior injection and adverse event is 25.5 days on average (highly variable range), with most occurring between three to six months after the first injection.3 No cases of occlusive vasculopathy after the first injection were reported in treatment-naïve patients. Of the 36 eyes that had an initial intraocular inflammatory event and continued treatment, 12 had a second adverse event. A basal incidence of 4.6 percent argues against such an unlucky independent recurrence. Interestingly, 36 to 52 percent of patients had anti-brolucizumab antibodies even before the study started, and approximately one quarter had boosted or new titers by study end. Patients with titers had a 6-percent incidence of IOI vs 2 percent in those without.10 Ana Bety Enriquez, MD, and colleagues and the Komodo registry identified female gender as an additional risk factor.6,7 Those risk factors drive general recommendations and contraindications for brolucizumab. In using the potent agent for patients with refractory progressive vision loss, patients should be aware of the known risk of IOI so far. Poor candidates are those with prior IOI or monocular patients. Brolucizumab is contraindicated in patients with active IOI. A careful dilated examination and return precautions should be performed before injection. For isolated anterior chamber inflammation, a widefield fluorescein angiogram, optical coherence tomography and treatment with topical steroids are reasonable. More aggressive intervention with systemic medications or intravitreal injection should be considered for intermediate or posterior segment involvement. Outcomes for vitrectomy haven’t revealed a benefit on visual acuity, but data so far data are scarce and skewed toward more severe IOI cases. So the decision remains up to individual discretion.11—H.N., M.S. |
 |
Bottom line
The association between brolucizumab and a heightened risk of IOI compared with other currently available anti-VEGF agents has been widely reported. Data from clinical trials and postmarket analyses have provided more information about who’s at greatest risk of IOI with brolucizumab, and the agent is contraindicated in patients with a history of IOI. Topical steroids are a reasonable first-line treatment for cases of anti-VEGF-induced IOI. RS
REFERENCES
1. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: Post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050-1059.
2. Singer M, Albini TA, Seres A, et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: Post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. Published online May 7, 2021. doi: 10.1016/j.oret.2021.05.003.
3. Dugel PU, Koh A, Ogura Y, et al; HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72-84.
4. Rahman EZ, Singer MA. Brolucizumab as treatment of wet age-related maculopathy. Drugs Today (Barc). 2020;56:699-704.
5. Daien V, Nguyen V, Essex RW, et al; Fight Retinal Blindness! Study Group. Incidence and outcomes of infectious and noninfectious endophthalmitis after intravitreal injections for age-related macular degeneration. Ophthalmology. 2018;125:66-74.
6. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139:441-448.
7. Singer M, Garweg J. Intraocular inflammation after anti-VEGF therapy: Latest data and guidance. Medscape Education CME activity. July 7, 2021. https://www.medscape.org/viewarticle/954171_2. Accessed August 23, 2021.
8. Novartis reports one year results of Phase III MERLIN study evaluating Beovu every four week dosing and provides update on Beovu clinical program [press release]. Basel, Switzerland. Novartis Media Relations; May 28, 2021. https://www.novartis.com/news/media-releases/novartis-reports-one-year-results-phase-iii-merlin-study-evaluating-beovu-every-four-week-dosing-and-provides-update-beovu-clinical-program. Accessed August 23, 2021.
9. Novartis Phase III Beovu data show potential for fluid resolution in more diabetic macular edema patients with fewer injections versus aflibercept [press release]. Basel, Switzerland. Novartis Media Relations; May 1, 2021. https://www.novartis.com/news/media-releases/novartis-phase-iii-beovu-data-show-potential-fluid-resolution-more-diabetic-macular-edema-patients-fewer-injections-versus-aflibercept. Accessed August 23, 2021.
10. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4:269-279.
11. Baumal C R, Bodaghi B, Singer MA, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5:519–527.



