 |
|
Bio Dr. Gopal is a second-year vitreoretinal surgical fellow at Mid Atlantic Retina/Wills Eye Hospital, Philadelphia Dr. Deaner specializes in uveitis and vitreoretinal surgery with Mid Atlantic Retina, Wills Eye Hospital, Philadelphia, and is an assistant professor of ophthalmology at Thomas Jefferson University, Philadelphia. Dr. Hsu is an attending physician at Mid Atlantic Retina and the Retina Service of Wills Eye Hospital. DISCLOSURES: Dr. Gopal has no relevant disclosures. Dr. Deaner is a consultant to Alimera Sciences, EyePoint Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Hsu has no relevant relationships to disclose. |
Her ocular history was notable for dry eye syndrome. She had photorefractive keratectomy for myopia years prior. Her medical history included hyperlipidemia, migraines and interstitial cystitis (IC), but she wasn’t on current treatment. She denied tobacco use. Family history was notable for a maternal grandmother with dry age-related macular degeneration. She was taking rosuvastatin and cyclobenzaprine.
Examination findings
Visual acuities were 20/200 OD and 20/20 OS. Intraocular pressures were normal in both eyes. Anterior segment evaluation demonstrated mild bilateral nuclear sclerotic cataracts. No anterior chamber or anterior vitreous inflammation were evident.
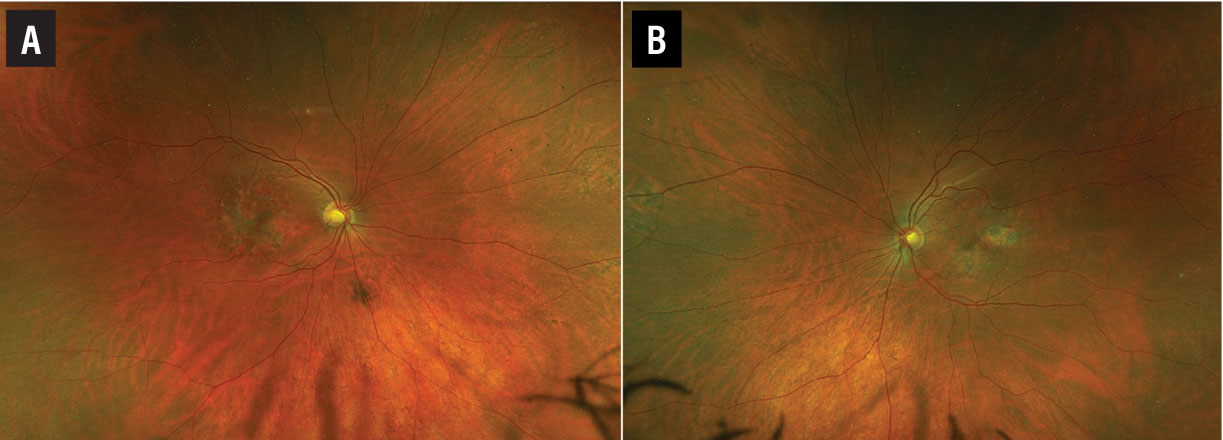
A fundus examination of the right eye (Figure 1A) revealed parafoveal pigment clumps with scattered yellowish fleck-like deposits interspersed with areas of retinal pigment epithelial atrophy. The fundus evaluation of the left eye (Figure 1B) demonstrated similar though less extensive findings with a prominent area of RPE atrophy in the temporal macula. The peripheral retina was unremarkable in both eyes.
Multimodal imaging
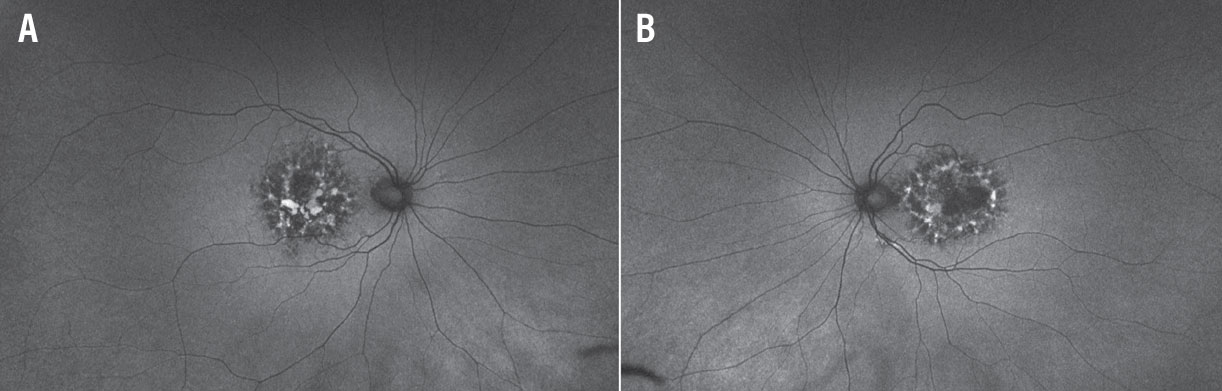
Fundus autofluorescence of both eyes (Figures 2A and 2B) revealed densely packed granular areas of intermixed hyperautofluorescence and hypoautofluorescence. Areas of hypoautofluorescence corresponding to areas of RPE atrophy were well-delineated.
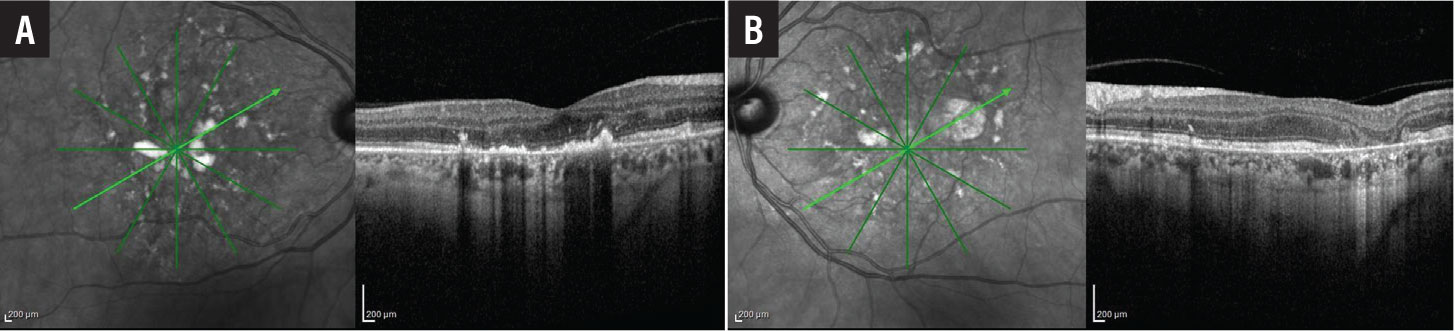
Near-infrared reflectance (NIR) imaging showed foci of hyperreflectivity and hyporeflectivity in both eyes (Figures 3A and 3B, page 15). On cross-sectional optical coherence tomography through the fovea of the right eye (Figure 3A page 15), we observed foci of nodular hyperreflectivity at the level of the RPE with disruption of overlying outer retinal laminations. The foveal contour was mildly irregular.
A few areas of hyperreflectivity were found in the outer nuclear layer. Inner retinal laminations were relatively well preserved. Cross-sectional OCT through the fovea of the left eye (Figure 3B, page 15) revealed similar hyperreflective deposits at the level of the RPE. There was loss of outer retinal layers in areas of RPE atrophy.
 |
|
Figure 1. Wide-field pseudocolor fundus imaging of the right (A) and left eyes (B) demonstrate parafoveal pigment clumping interspersed with yellow subretinal deposits and areas of retinal pigment epithelial atrophy. |
 |
|
Figure 2. Wide-field fundus autofluorescence imaging of the right (A) and left (B) eyes reveals densely packed granular areas of intermixed hyperautofluorescence and hypoautofluorescence. |
Additional history and diagnosis
Considering these imaging findings, further interrogation of this patient’s medication record disclosed a six-year history of pentosan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals) use at a dose of 100 mg t.i.d. She had stopped pentosan three years before onset of her visual symptoms for reasons unrelated to retinal toxicity.
Given her clinical history and constellation of multimodal imaging findings, she was diagnosed with pentosan polysulfate maculopathy (PPSM). She was counseled on Amsler grid monitoring. At follow-up, six months later, the RPE atrophy in both eyes showed mild progression on OCT. Her visual acuity had decreased to 20/400 in the right eye with otherwise stable visual acuity in the left eye.
Pentosan polysulfate maculopathy
PPSM is a recently described clinical entity, first reported in 2018 in a series published by Nieraj Jain, MD, and colleagues describing a novel pigmentary maculopathy observed in six patients with chronic exposure to PPS.1 Despite recent recognition of its potentially toxic effects on the retina, PPS was described structurally first in the 1950s and has been used off-label for various clinical and veterinary indications since the 1980s.2
The U.S. Food and Drug Administration approved pentosan in 1996 under the brand name Elmiron for the relief of bladder pain and discomfort associated with IC.3 IC is a chronic lower urinary tract pain syndrome of unknown etiology characterized by bladder pain, urinary frequency and urgency.3 Urine cultures and other infectious laboratory investigations are negative in these patients. Adult women comprise the vast majority of affected patients. Because of its chronic nature, IC can be functionally debilitating.
Management is symptomatic and often multiarmed, and includes a combination of lifestyle modification, anticholinergic drugs, neuromodulating agents and intravesicular therapy.3 As the only FDA-approved oral agent indicated for IC, PPS is a convenient first- and second-line therapeutic option for patients with IC.
Molecularly, PPS is a semisynthetic heparin-like sulfated polysaccharide. While its exact mechanism of action in IC is uncertain, it’s been postulated to coat the glycosaminoglycan layer lining the uroepithelium, reducing bladder wall permeability and offering a mechanical barrier to the irritating effects of urinary toxins.3
The role of PPS in the development of retinal toxicity isn’t well elucidated, but authors have hypothesized that it interferes with RPE-photoreceptor homeostasis with resultant aberrant processing of photoreceptor outer segments.2
 |
|
Figure 3. A) Optical coherence tomography of the right eye shows multiple foci of nodular hyperreflectivity at the level of the retinal pigment epithelium with disruption of overlying outer retinal laminations. B) OCT of the left eye shows similar findings with focal loss of the outer retina corresponding to areas of retinal pigment epithelial atrophy. |
Clinical and imaging markers of PPSM
Symptomatically, patients with PPSM may present with nyctalopia, prolonged dark adaptation, difficulty reading and metamorphopsia.4 Central visual acuity is typically preserved in early stages, but declines with progressive disease.5
Fundoscopically, patients demonstrate hyperpigmented spots in the macula with yellowish subretinal deposits. Patchy areas of paracentral RPE atrophy may accompany more advanced disease with associated central acuity decline if the fovea is involved. Macular findings may be subtle and asymmetric early on. Detection in such cases relies on the use of retinal imaging.
On FAF, eyes with PPSM demonstrate a strikingly dense reticular array of hyperautofluorescent and hypoautofluorescent signal in the parafoveal region. A peripapillary hypoautofluorescent halo may also be noted in cases where the disease extends near the optic disc, which may help distinguish PPSM from other retinal dystrophies.
OCT reveals nodular hyperreflective foci at the level of the RPE with associated shadowing of the underlying choroid, which appear to correspond to the pigmented spots on fundus evaluation. Areas of retinal thinning and RPE atrophy can be readily visualized. No accepted OCT correlate exists for the yellow deposits seen on clinical exam.2
Differential diagnosis
The differential diagnosis of PPSM includes AMD, pattern dystrophies, chronic central serous retinopathy, pachychoroid pigment epitheliopathy and maternally inherited diabetes and deafness (MIDD) syndrome.2 Despite a spectrum of fundoscopic appearance, PPSM can be readily differentiated from other etiologies of pigmentary maculopathy in the appropriate clinical context with the aid of characteristic findings on multimodal imaging.
Risk assessment and monitoring
Risk of retinal toxicity with PPS exposure occurs in a dose-dependent fashion, typically over several years with greater cumulative dose. An electronic health records study within a comprehensive health system identified a PPSM prevalence rate of 12.7 percent in patients with a cumulative PPS dose in the 500-to-999-g range that increased to 41.7 percent in patients exposed to a >1,500-g cumulative dose.6
PPSM may progress even after drug discontinuation, as we observed in our patient. Patients are at risk for additional vision-threatening complications, including geographic atrophy, cystoid macular edema and choroidal neovascularization. They should be monitored for development of such.2
No treatment exists for PPSM, highlighting the importance of routine screening and prompt recognition of retinal toxicity in patients prescribed PPS.
In June 2020, the FDA updated the label for PPS in the wake of mounting postmarket evidence of an associated drug-induced pigmentary maculopathy, recommending baseline fundus exam, OCT and FAF within six months of starting therapy and periodically thereafter.7
Soon after, the American Urogynecologic Society released a practice advisory recommending prescribers counsel patients on the possible visual effects of PPS prior to initiation, limit dose and duration of exposure when possible, and help coordinate baseline and routine screening retinal evaluation.8 To date, many individuals likely remain undiagnosed or misdiagnosed.
Bottom line
PPSM is a recently described pigmentary maculopathy with potentially visually debilitating effects. Long-term exposure to PPS increases the risk of retinal toxicity, highlighting the importance of patient education, dose monitoring and regular retinal evaluation. Characteristic findings on OCT, NIR and FAF imaging are key in early diagnosis. Further research is needed to better understand the pathophysiology and clinical phenotype of this disease to better aid surveillance and early detection. RS
REFERENCES
1. Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology. 2018;125:1793-1802.
2. Lindeke-Myers A, Hanif AM, Jain N. Pentosan polysulfate maculopathy. Surv Ophthalmol. 2022;67:83-96..
3. Anderson VR, Perry CM. Pentosan polysulfate: A review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs. 2006;66:821-35.
4. Hanif AM, Armenti ST, Taylor SC, et al. Phenotypic spectrum of pentosan polysulfate sodium-associated maculopathy: A multicenter study. JAMA Ophthalmol. 2019;137:1275-1282.
5. Jain N, Liao A, Garg SJ, et al, for the Macula Society Pentosan Polysulfate Maculopathy Study Group. Expanded clinical spectrum of pentosan polysulfate maculopathy: A Macula Society Collaborative Study. Ophthalmol Retina. 2022;6:219-227.
6. Vora RA, Patel AP, Melles R. Prevalence of maculopathy associated with long-term pentosan polysulfate therapy. Ophthalmology. 2020;127:835-836.
7. U.S. Food and Drug Administratoin. Drug Safety-related Labeling Changes (SrLC) Database Overview: Pentosan polysulfate sodium. Updated March 12, 2021. https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2277.
8. 2020 Practice Advisory: Possible drug induced retinal maculopathy secondary to long-term use of pentosan polysulfate sodium. American Urogynecologic Society. https://www.augs.org/practice-advisory-possible-drug-induced-retinal-maculopathy-secondary-to-long-term-use-of-pentosan-polysulfate-sodium-elmiron/.




