Innovations in Retina Surgery
Take-home points
|
 |
|
Bios Dr. Karani is a vitreoretinal surgery fellow at the Wilmer Eye Institute of Johns Hopkins University in Baltimore. Dr. Kashani is the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University. DISCLOSURES: Dr. Karani has no relevant disclosures. Dr. Kashani is a consultant to Carl Zeiss Meditec and received honoraria and grant support from Carl Zeiss Meditec. |
Optical coherence tomography is an essential tool in the modern-day retina practice. Since its conception three decades ago, OCT technology has evolved from having limited use in the clinical setting to being used in the diagnosis of almost every retinal disease.1 OCT has been developed further to be used in the anterior segment of the eye, and even in fields such as gastroenterology and neurology.2,3
One of the most recent applications of OCT technology is its use in guiding intraocular retinal surgery. This article discusses the practical application of microscope-integrated intraoperative OCT in the retina practice and the types of cases for which it is most useful.
Evolution of iOCT
An early attempt at using handheld OCT in the perioperative setting, separately from an exam under anesthesia, was to preoperatively and postoperatively scan the macula after epiretinal membrane and internal limiting membrane peeling.4 The surgeons wrapped the handheld OCT scanner in a sterile covering. The surgeon controlled it while an assistant helped with the software.
Several other studies demonstrated the potential utility of using OCT intraoperatively in other areas, such as guiding membrane peel procedures and in analyzing subretinal fluid in retinal detachment repairs.5 However, handheld iOCT had a number of limitations, among them the need for precise patient positioning; user fatigue and motion, leading to artifacts during scanning; and the demand for a surgical pause when using the device.6
The introduction of microscope-mounted OCTs, the next iteration of iOCT, improved upon problems with scan quality and positioning by giving the surgeon precise OCT control with a foot pedal. This also improved image quality and reproducibility over handheld operation.
A prospective, case-controlled series using the Bioptigen SDOIS portable OCT on a custom-designed microscope-mounted system showed that iOCT imaging was feasible in 518 of 531 eyes (98 percent).7 A survey of the involved surgeons found that iOCT aided in surgical decision-making in 48 percent of anterior segment procedures and 43 percent of retina cases.
The most frequent procedures for which iOCT impacted decision-making were Descemet stripping automated endothelial keratoplasty (DSAEK) and deep anterior lamellar keratoplasty among anterior segment procedures, and pars plana vitrectomy with epiretinal membrane peeling and macular hole cases.7
 |
Latest iteration of iOCT
The most recent and practical iteration of iOCT is the microscope-integrated iOCT. These OCT systems are integrated within the surgical microscope, allowing for surgeons to receive almost real-time feedback during a procedure without the need to stop the case or perform laborious manipulations of a handheld unit in a sterile setting.
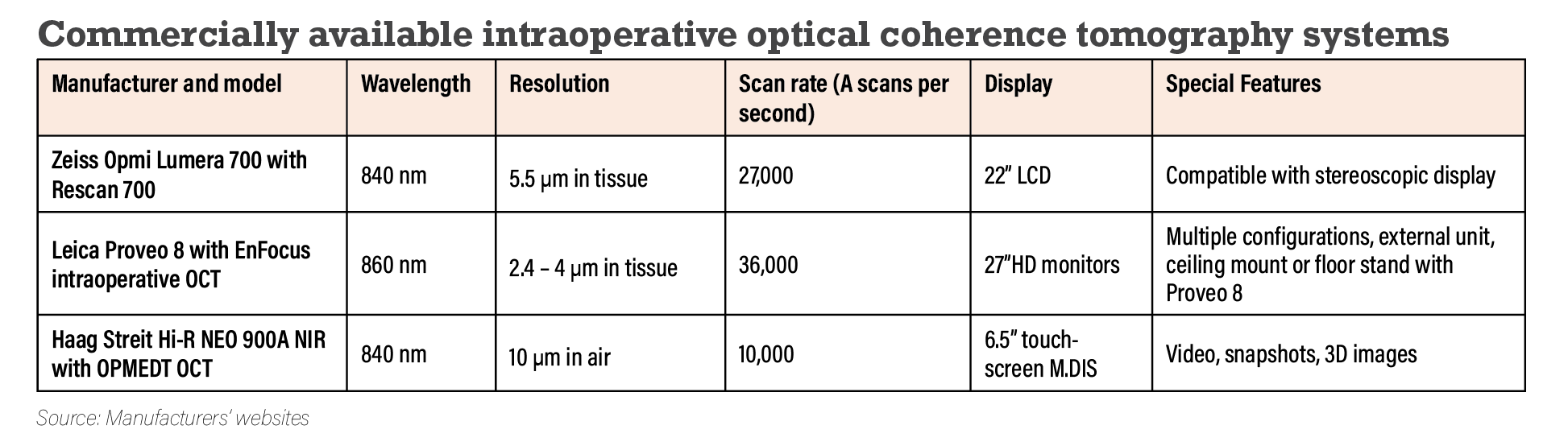
A single-site, three-year, multisurgeon, prospective case series assessed the utility and feasibility of microscope-integrated iOCT for anterior and posterior segment procedures.8 Patients undergoing surgery were imaged using one of three iOCT systems—Leica EnFocus, Zeiss Rescan 700 or Haag-Streit iOCT (Table). An image was obtained in 820 of 837 study eyes (98 percent), demonstrating the feasibility of iOCT.
Participating surgeons indicated that iOCT impacted decision-making in 29 percent of posterior segment cases and 43 percent of anterior segment cases.8,9 Specifically, decision making utility was found to be most impactful in DSAEK and Descemet membrane endothelial kerotoplasty among anterior segment procedures, and membrane peeling, macular hole and proliferative vitreoretinopathy cases among posterior segment cases.8
iOCT indications: Vitreomacular interface disorders
Visually significant vitreomacular interface disorders often require surgical management with PPV, and ERM or ILM peel.10 Intraoperative OCT has potential in these cases because it allows the surgeon to find appropriate planes for peeling and areas of focal damage, as well as assess the vitreomacular interface intraoperatively after performing a peel procedure.
• Macular hole closure. This procedure often involves PPV with ILM peel and gas tamponade with posturing. The retinal architecture after membrane peeling is not well understood and discussion continues over the use of gas tamponades and how long patients should posture after surgery with gas.11
A 2019 study of 37 patients showed that iOCT markers could be used to create a predictive model for macular hole closure, and help to prognosticate post-op vision and improve post-op posturing times.12 A 2021 study of 10 eyes assessing macular hole closure during surgery using iOCT showed a decrease in minimal aperture macular hole diameter.13
As iOCT technology becomes more refined, we can continue to use it to more accurately visualize real-time effects of ILM peeling on macular hole closure.
• Vitreomacular traction. VMT release often requires PPV and ILM peeling, during which the underlying retinal tissues can become elevated or incur damage. A 2015 study of 163 patients showed that iOCT could demonstrate damage to the nerve fiber layer during ILM peeling. Most of these changes occurred from direct instrument contact and from indirect traction resulting from the membrane peel.14
• Epiretinal membrane. Treatment of ERM often involves both ERM and ILM peel. Classically, surgeons use ILM stains such as indocyanine green and Brilliant Blue G to help identify membranes. In a 2022 study of 56 eyes, half of the eyes underwent membrane peel without iOCT and half with it.
In the eyes that had membrane peel without iOCT, only 25 percent of surgeons reported they were able to peel without stain; the remaining 75 percent required stain. In the iOCT group, about 93 percent of membranes could be peeled without staining; only about 8 percent required staining.
These results showed that iOCT can help assess epiretinal membrane removal, perhaps as efficaciously as dyes used for staining.15 iOCT illustrations (Figure 1) readily demonstrated change in the conformation of the retina and membrane before, during and after the membrane peel. This is particularly illustrative for trainees who may not be able to easily visualize these subtle changes. In addition, both experienced surgeons and trainees may use iOCT to confirm the boundaries of the peel and guide subsequent steps in the peeling procedure.
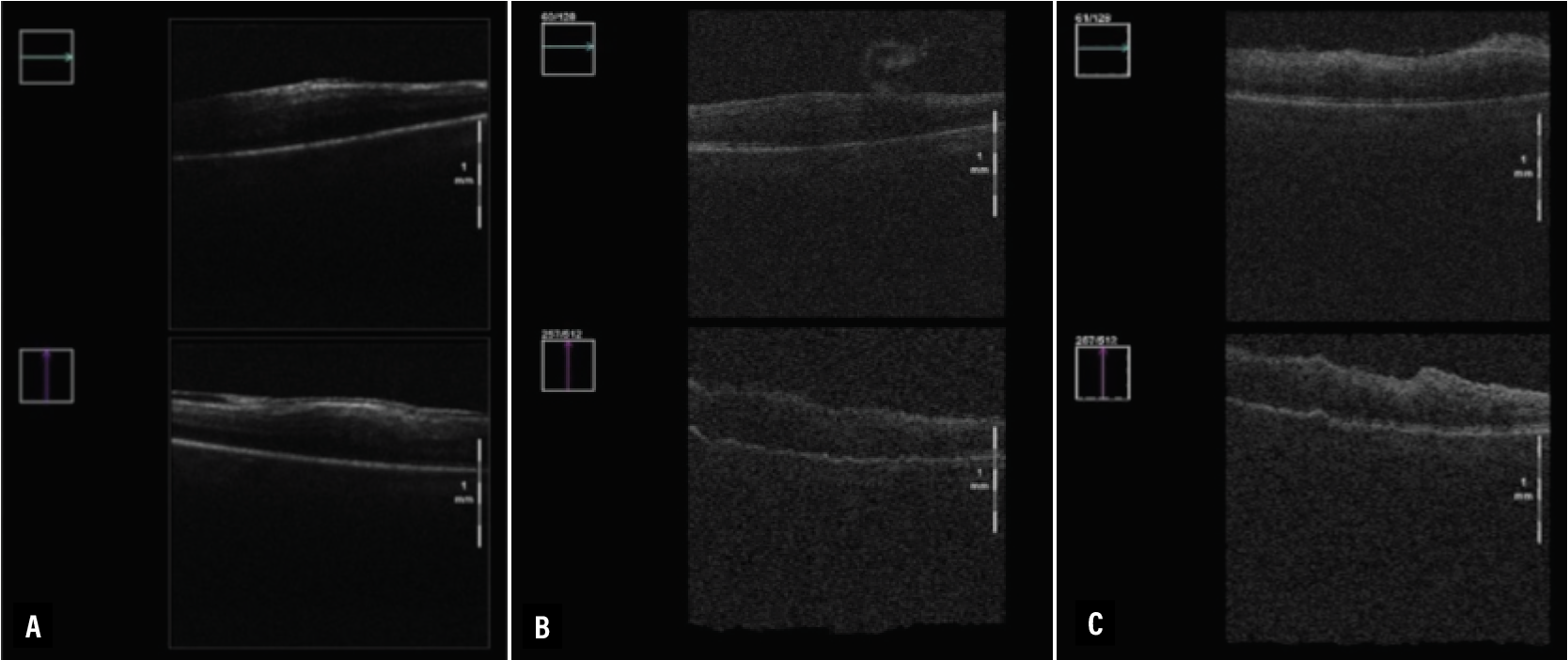 |
| Figure 1. Illustration of intraoperative optical coherence tomography images before (A), during (B) and after (C) a case for membrane peeling highlight salient anatomic changes. |
Tractional retinal detachments
TRDs and combined rhegmatogenous and tractional retinal detachments are a common complication in patients with proliferative diabetic retinopathy. Surgical techniques for these patients rely on creating tissue planes to dissect tractional membranes from the retina.
Current approaches to these detachments involve using the vitrector, retinal forceps and retinal scissors to dissect membranes. These techniques are effective, but accurately determining the tissue planes, adherence points and iatrogenic retinal breaks can be challenging.16
A 2018 study highlighted the utility of iOCT for treatment of TRD in PDR.17 iOCT was successfully obtained in 80 of 81 study eyes (99 percent). Surgeons reported that in 51 percent of cases, iOCT provided valuable surgical information. In 26 percent of the cases, iOCT altered surgical decision-making, particularly in determining the presence of retinal breaks and the need for more membrane peeling.17
Two similar studies demonstrated that iOCT helped identify areas of fibrovascular adhesion and retinal holes, and helped decrease the amount of surgical instrumentation needed to create tissue planes.18,19 However, one limitation of iOCT in patients with PDR is preretinal hemorrhage.17
Many PDR patients have non-clearing vitreous hemorrhage before surgery, which makes preoperative OCT impractical. In these cases, iOCT allows for detailed assessment of the retina. As the technology becomes more widespread, surgical techniques are being developed around this modality to help facilitate these types of procedures.
Rhegmatogenous retinal detachments
RRD repairs are among the most common procedures vitreoretinal surgeons do. In a 2013 series of nine eyes that had PPV for RRD repair, microscope-mounted iOCT showed foveal architecture changes in all patients.20 The changes varied among patients, but included foveal thinning, foveal contour loss, inner retinal thinning and macular hole.
A separate study of nine patients in 2015 demonstrated persistent occult subretinal fluid after air-fluid exchange in all eyes, and further supported the rationale for face-down positioning in patients undergoing RRD repair and gas tamponade.21
A potential area of study for iOCT use could be to intraoperatively assess drainage success in patients having scleral buckle with drainage retinotomy for RRD repair. iOCT has the potential to further determine intraoperative microscopic changes that occur during retinal detachment repair. Its uses in this procedure are still being elucidated.
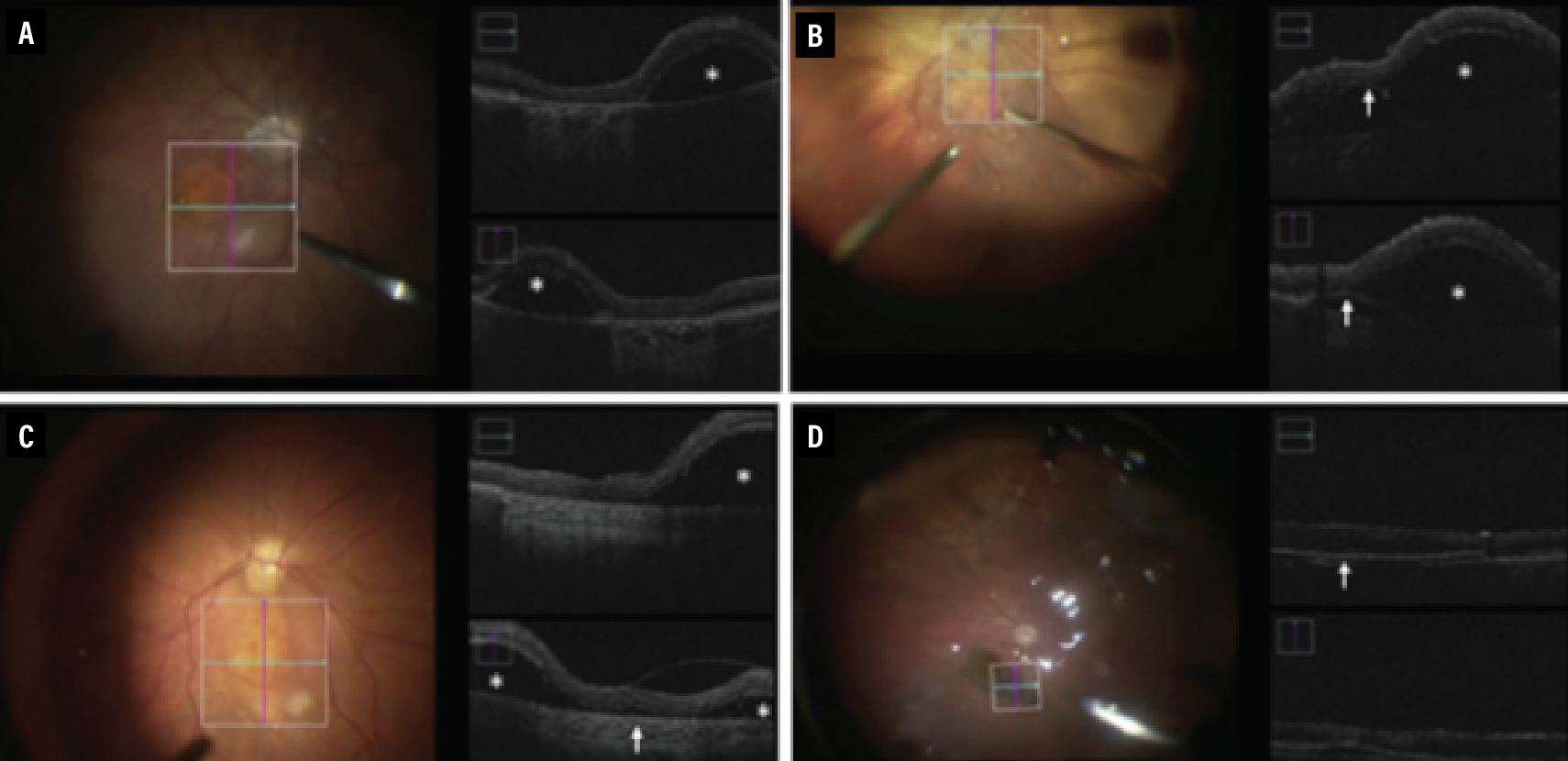 |
| Figure 2. Intraoperative optical coherence tomography images from subretinal placement of CPCB-RPE1, a stem cell-derived retinal pigment epithelium monolayer seeded on a synthetic substrate. A) Bleb formation. B) Focal area of RPE detachment. C) Retinal adhesion to Bruch’s membrane within the area of geographic atrophy. D) Flattening of the bleb after Perfluoro-n-octane injection and location of CPCB-RPE1 after administration. (Reproduced under Creative Commons License from Kashani AH, Uang J, Mert M, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a Phase 1/2a study. Ophthalmol Retina. 2020;4:264-273.) |
Subretinal therapy
A particularly novel application of iOCT is in the space of subretinal surgery to treat inherited retinal diseases and nonexudative age-related macular degeneration. Current subretinal therapies involve injection of stem cell-derived tissues or transgenes in viral vectors underneath the retina.22 While most subretinal therapies are currently in clinical trial phase, the Food and Drug Administration in 2019 approved voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics) as a subretinal gene therapy for biallelic RPE65 mutations.23 This therapy is exclusively delivered with a subretinal bleb that can be monitored with iOCT. 24
Another group evaluated implantation of a stem cell-derived RPE monolayer seeded on a synthetic substrate (CPCB-RPE1). This showed the potential utility of iOCT in preparing for and confirming stem cell implant location.22 The study involved implanting an approximate 3-x-6-mm sheet of stem cell-derived RPE into an area of geographic atrophy in patients with advanced non-exudative AMD.
PPV was performed and a subretinal pocket was created using a targeted hydrodissection technique encompassing the area of GA with a 1 disc diameter margin for 360 degrees. iOCT was used to assess the lateral dimensions and depth of the subretinal fluid pocket. Then, the implant was injected through a retinotomy into this subretinal pocket.
iOCT was used at several key points throughout the procedure, including before bleb formation to assess the area of GA, after bleb formation to assess bleb size, after implantation to determine implant orientation, and after flattening of the subretinal pocket with PFO (Figure 2). iOCT was used in nine of 16 enrolled subjects, and it proved useful in determining areas of incomplete subretinal hydrodissection to create a subretinal pocket, in identifying incorrect implant placement and in dissecting subretinal adhesions to Bruch’s membrane.
Several studies have explored the utility of iOCT in the subretinal delivery of transgenes on viral vectors.25 The largest study in this area is a 2020 study involving patients enrolled in various subretinal gene therapy trials, including 19 eyes in which subretinal blebs were created.23 The participating surgeons used sharp or blunt needle tips to create a subretinal bleb, based on their discretion, and iOCT to determine the difference between the two different needle techniques. iOCT visualized medication injection, medication reflux and retinotomy size (Figure 3).
While iOCT is useful in subretinal procedures, certain limitations have been highlighted in its use. These include a relatively small field of view, operative site shadowing with surgical instruments and the need for training to become skilled with the technology.25 As subretinal surgery techniques are refined, the role of iOCT in this arena will continue to evolve.
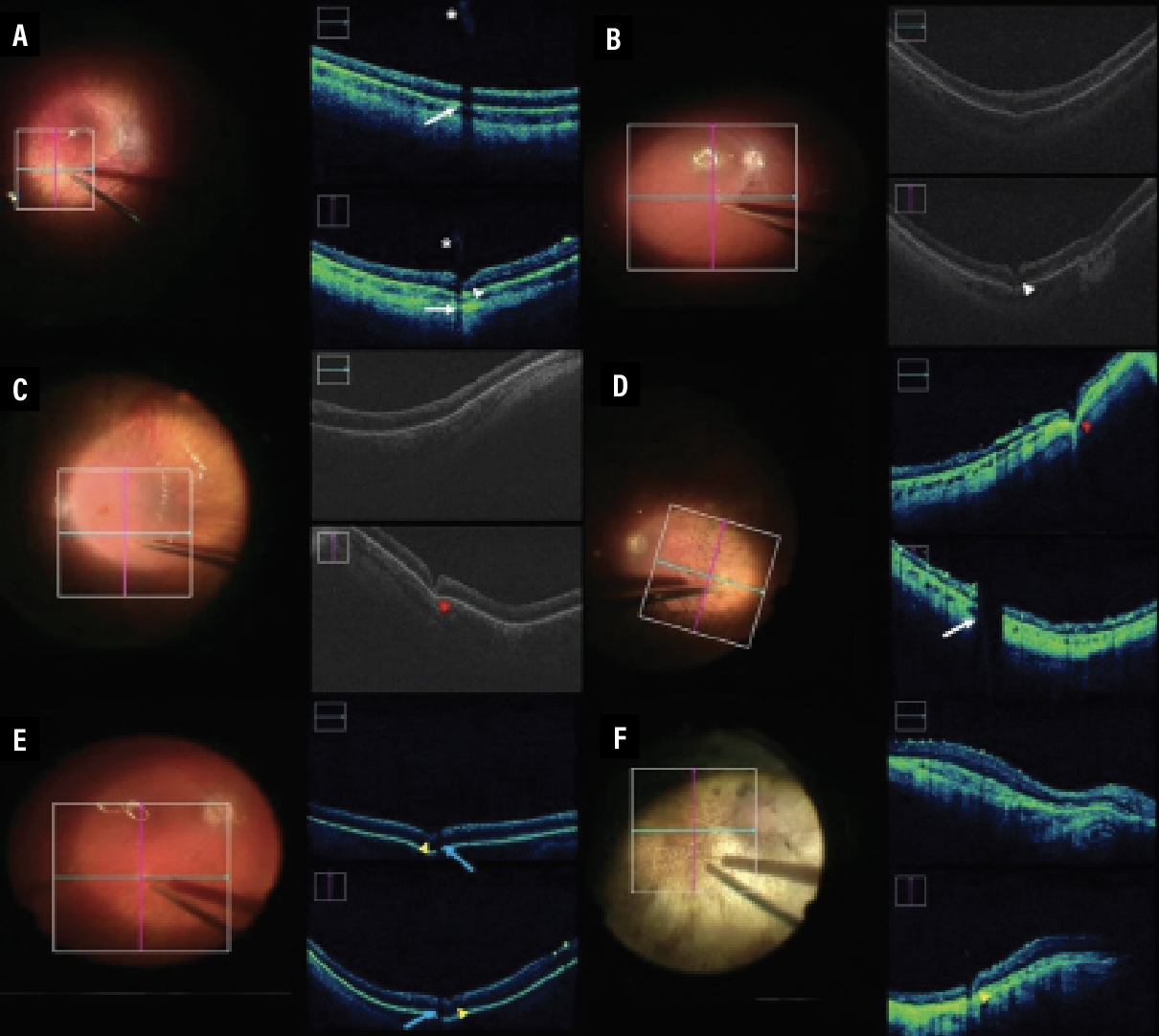 |
| Figure 3. Intraoperative optical coherence tomography images obtained during gene therapy surgery. A,B) Unsuccessful initial step of subretinal injection with blunted needle with corresponding shadow of subretinal cannula. C,D) Successful subretinal injection with blunted needle with corresponding shadow of subretinal cannula. E,F) Successful subretinal injection with sharp needle with corresponding shadow of subretinal cannula are demonstrated. (Reproduced under Creative Commons Attribution License from Vasconcelos HM Jr, Lujan BJ, Pennesi ME, Yang P, Lauer AK. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int J Retina Vitreous. 2020;6:13.) |
Other subretinal procedures
Intraoperative OCT has also been used in subfoveal Perfluoro-n-octane (PFO) removal and in subretinal tissue plasminogen activator (tPA) injection.
• PFO removal. PFO entrapment is a rare but potentially serious complication of using PFO in retinal detachment surgery. We commonly use it to flatten the retina and to displace subretinal fluid. Entrapment of PFO under the retina can manifest as a scotoma, which can cause visual impairment when present under the fovea.
Symptomatic PFO entrapment requires surgical removal, but there’s no commonly accepted way of approaching this problem. Surgical approaches include using a 41-gauge needle to aspirate the PFO bubble or a 38-gauge macular hydrodissection cannula, both of which are transretinal.26–28 These approaches all involve precise preoperative planning using OCT and don’t allow direct visualization of the PFO removal process.
With iOCT, the surgeon can directly visualize the needle as it approaches the bubble and avoid damage to nearby structures, as well as ensure the bubble has been removed intraoperatively. A 2015 case report showed successful use of the iOCT to remove PFO using a 41-gauge needle (Figure 4).29
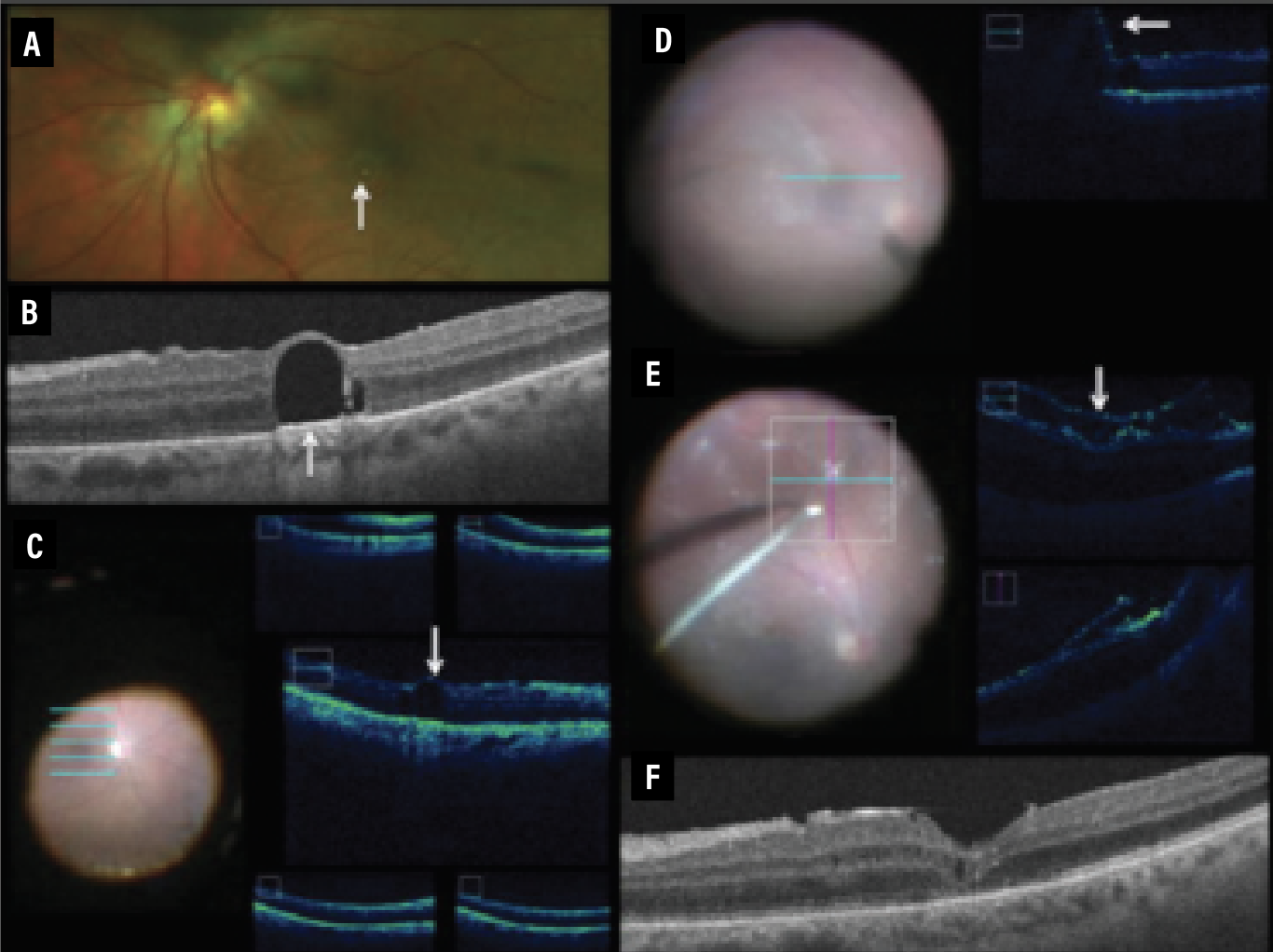 |
| Figure 4. Intraoperative optical coherence tomography for subretinal Perfluoro-n-octane removal. A) Macular photo shows chronic retained subfoveal PFO. B) Preoperative OCT of subretinal PFO. C) Five-line raster scan depicting subretinal fluid at the beginning of a case. D) One-line raster scan depicting subretinal bleb induction with 41-gauge cannula. E) iOCT horizontal and vertical scan demonstrating detached retina, proliferative vitreoretinopathy and Triesence staining. F) OCT at three months postoperatively shows the absence of PFO, inner/outer segment junction abnormalities and trace macular edema. |
• tPA injection. Significant submacular hemorrhage is a devastating complication of nAMD. Dense hemorrhage is often difficult to clear with standard anti-VEGF injections, and can cause photoreceptor loss and subretinal fibrosis. In such cases, tPA injection has been shown to liquify and help displace the hemorrhage when coupled with a gas bubble.
 |
This procedure involves creating a retinotomy with a 41-gauge cannula, injection of the tPA in the subretinal space and fluid-air exchange. Similar to PFO removal, current techniques don’t allow for direct visualization of the subretinal procedure and of confirmation of tPA injection.30 A 2015 case used a microscope-mounted iOCT to confirm appropriate localization of tPA injection.30 While this was the first report of this technique, advancements in technology and more widespread availability of iOCT will likely increase use of this procedure.
Bottom line
Retinal surgery is all about visualization, and iOCT allows us to visualize the retina during surgery in previously unseen detail and dimension. Capitalizing on this ability will allow us to make more informed decisions during surgery and ultimately lead to better outcomes for patients.
Intraoperative OCT technology will continue to advance and find different roles in vitreoretinal surgery and will ultimately allow surgeons to perform safer and more efficient retinal procedures, while simultaneously allowing us to glean more knowledge about the retina as it undergoes surgery. RS
REFERENCES
1. Gabriele M, Wollstein G, Ishikawa H, Kagemann L, et al. Optical coherence tomography: History, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52:2425-2436.
2. Tsai TH, Leggett CL, Trindade AJ, Sethi A, et al. Optical coherence tomography in gastroenterology: A review and future outlook. J Biomed Opt. 2017;22:1-17.
3. Maldonado RS, Mettu P, El-Dairi M, Bhatti MT. The application of optical coherence tomography in neurologic diseases. Neurol Clin Pract. 2015;5:460-469.
4. Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery [Published correction appears in Retina. 2011;31:1236]. Retina. 2009;29:1457-1468.
5. Hahn P, Migacz J, O'Connell R, Maldonado RS, et al. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011;42 Suppl:S85-S94.
6. 6. Jayadev C, Dabir S, Vinekar A, Shah U, Vaid T, Yadav NK. Microscope-integrated optical coherence tomography: A new surgical tool in vitreoretinal surgery. Indian J Ophthalmol. 2015;63:399-403.
7. Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic Imaging with Optical Coherence Tomography (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014;158:999-1007.
8. Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology. 2018;125:1014-1027.
9. Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: The DISCOVER Study RESCAN results. JAMA Ophthalmol. 2015;133:1124-1132.
10. Levison, AL and Kaiser, PK. Vitreomacular interface diseases: diagnosis and management. Taiwan J Ophthalmol. 2014;4:63-68.
11. Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK. Intrasurgical dynamics of macular hole surgery: An assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34:213-221.
12. Ehlers JP, Uchida A, Srivastava SK, Hu M. Predictive model for macular hole closure speed: insights from intraoperative optical coherence tomography. Trans Vis Sci Tech. 2019;8:18.
13. Nishitsuka K, Nishi K, Namba H, Kaneko Y, Yamashita H. Intraoperative observation of a macular holes using optical coherence tomography. Clin Optom (Auckl). 2021;13:113-118.
14. Ehlers JP, Han J, Petkovsek D, Kaiser PK, et al. Membrane peeling-induced retinal alterations on intraoperative oct in vitreomacular interface disorders from the PIONEER study. Invest Ophthalmol Vis Sci. 2015;56:7324-7330.
15. Lorusso M, Micelli Ferrari L, Gisotti EN, et al. Success of iOCT in surgical management of ERM peeling. Eur J Ophthalmol. 2022;32:3116-3120.
16. Khan M, Ehlers JP. Clinical utility of intraoperative optical coherence tomography. Curr Opin Ophthalmol. 2016;27:201-209.
17. Khan M, Srivastava SK, Reese JL, Shwani Z, Ehlers JP. Intraoperative OCT-assisted surgery for proliferative diabetic retinopathy in the DISCOVER study. Ophthalmol Retina. 2018;2:411-417.
18. Agarwal A, Gupta V. Intraoperative optical coherence tomography and proportional reflux hydrodissection-guided pars plana vitrectomy for complex severe proliferative diabetic retinopathy. Indian J Ophthalmol. 2020;68:177-181.
19. Gabr H, Chen X, Zevallos-Carrasco OM, et al. Visualization from intraoperative swept-source microscope-integrated optical coherence tomography in vitrectomy for complications of proliferative diabetic retinopathy. Retina. 2018;38 Suppl 1:S110-S120.
20. Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33:1428-1434.
21. Toygar O, Riemann CD. Intraoperative optical coherence tomography in macula involving rhegmatogenous retinal detachment repair with pars plana vitrectomy and perfluoron. Eye (Lond). 2016;30:23-30. doi:10.1038/eye.2015.230.
22. Kashani AH, Uang J, Mert M, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a Phase 1/2a study. Ophthalmol Retina. 2020;4:264-273.
23. Vasconcelos HM Jr, Lujan BJ, Pennesi ME, Yang P, Lauer AK. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int J Retina Vitreous. 2020;6:13.
24. Ameri H. Prospect of retinal gene therapy following commercialization of voretigene neparvovec-rzyl for retinal dystrophy mediated by RPE65 mutation. J Curr Ophthalmol. 2018;30:1-2.
25. Ladha R, Caspers LE, Willermain F, de Smet MD. Subretinal therapy: Technological solutions to surgical and immunological challenges. Front Med (Lausanne). 2022;9:846782.
26. García-Arumí J, Castillo P, López M, et al. Removal of retained subretinal perfluorocarbon liquid. Br J Ophthalmol. 2008;92:1693-1694.
27. Ghasemi Falavarjani K, Anvari P. Surgical removal of submacular perfluorocarbon liquid using a 41-gauge extendible subretinal injection needle. J Ophthalmic Vis Res. 2019;14:393-397.
28. Hanout M, Muni RH. Novel surgical technique to remove retained subfoveal perfluorocarbon liquid. Retin Cases Brief Rep. 2021;15:741-744.
29. Smith AG, Cost BM, Ehlers JP. Intraoperative OCT-assisted subretinal perfluorocarbon liquid removal in the DISCOVER study. Ophthalmic Surg Lasers Imaging Retina. 2015;46:964-966.
30. Ehlers JP, Petkovsek DS, Yuan A, Singh RP, Srivastava SK. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46:327-332.



