Take-home points
|
 |
|
Bios Dr. Kasetty is a post-graduate year four resident at Henry Ford Hospital in Detroit. Ms. Baines is a fourth-year medical student at Texas Tech Health Sciences Center El Paso, Paul L. Foster School of Medicine. Dr. Marcus is a vitreoretinal surgeon and medical director at Southeast Retina Center, PC, director of clinical research at Eye Health America and professor of clinical ophthalmology, Medical College of Georgia, Augusta DISCLOSURES: Dr. Kasetty, Ms. Baines have no relevant disclosures. Dr. Marcus disclosed relationships with RegenxBio, Genentech/Roche, Regeneron Pharmaceuticals, Clearside Biomedical, Annexon Biosciences, Apellis Pharmaceuticals, Vial, Coherus BioSciences, Vantage Biosciences, Amgen, Ionis, Xplore, Mylan, Opthea, Iveric Bio, Outlook Therapeutics, Hengenix, Graybug Vision, Topcon, Gyroscope Therapeutics, Roche, Xplore, Kodiak Sciences, Oculis, Alexion and Ocular Therapeutix. |
When the Diabetic Retinopathy Severity Score was first established more than 30 years ago, it was based on imaging findings predominantly in the posterior pole, so the mid-peripheral and peripheral retina typically weren’t imaged.1 Advancements in retinal imaging, such as ultra-widefield imaging, have enabled capturing more retinal area in a single image,2 but the DRSS hasn’t been updated to account for these imaging modalities.
To help incorporate these imaging advancements, the DRCR Retina Network initiated Protocol AA, a four-year multicenter prospective observational study to evaluate the ability of UWF fundus photography and fluorescein angiography in assessing the risk of retinopathy progression in treatment-naïve nonproliferative diabetic retinopathy eyes. Here, we discuss the findings of DRCR Protocol AA and its applicability to clinical practice.
From posterior pole to the periphery
Since 1991, the DRSS from the Early Treatment Diabetic Retinopathy Study has been the established system for grading the severity and progression of diabetic retinopathy in clinical trials and epidemiologic studies.
The ETDRS used film seven-standard field fundus photos that captured approximately 30 to 35 percent of the retinal area, predominantly capturing the posterior pole.1 Previous DRCR Retina Network studies validated agreement between the DRSS clinical exam and film seven-standard field fundus photos, as well as between digital and film seven-standard field images.3,4
UWF imaging, centered on the fovea extending anteriorly to the vortex veins in all four quadrants, captures approximately 82 percent of the retinal area, including the peripheral retina (Figure 1).5 Thus, UWF imaging allows us to identify peripheral lesions outside the standard EDTRS fields and better assess DR lesions.
Additionally, UWF fluorescein angiography allows us to identify peripheral nonperfusion, microaneurysm leakage, neovascularization and vascular leakage.2
When diabetic lesions, such as hemorrhages, microaneurysms, intraretinal microvascular abnormalities and venous beading, are located primarily outside the standard EDTRS seven-field photos, they’re termed predominantly peripheral lesions.6 Previous small and short-term studies have demonstrated that PPL may be predictive of higher risk of DR progression.2,7-13
UWF color and FA PPLs
DRCR Protocol AA enrolled 388 patients and analyzed 544 eyes. Forty-one and 46 percent of eyes had PPLs on baseline UWF color photos and UWF FA, respectively. The most common PPLs were hemorrhages/microaneurysms.
Of the 542 eyes with gradable UWF color and FA photography, 16, 20 and 25 percent had PPLs present on color photos, FA, and both color photos and FA, respectively. Therefore, grading of PPL on UWF FA and color photos was discordant. Thirty-nine percent of eyes had no PPLs on either UWF color photos or FA. The most common location for PPLs were in Fields 3, 4 and 6 (Figure 1).
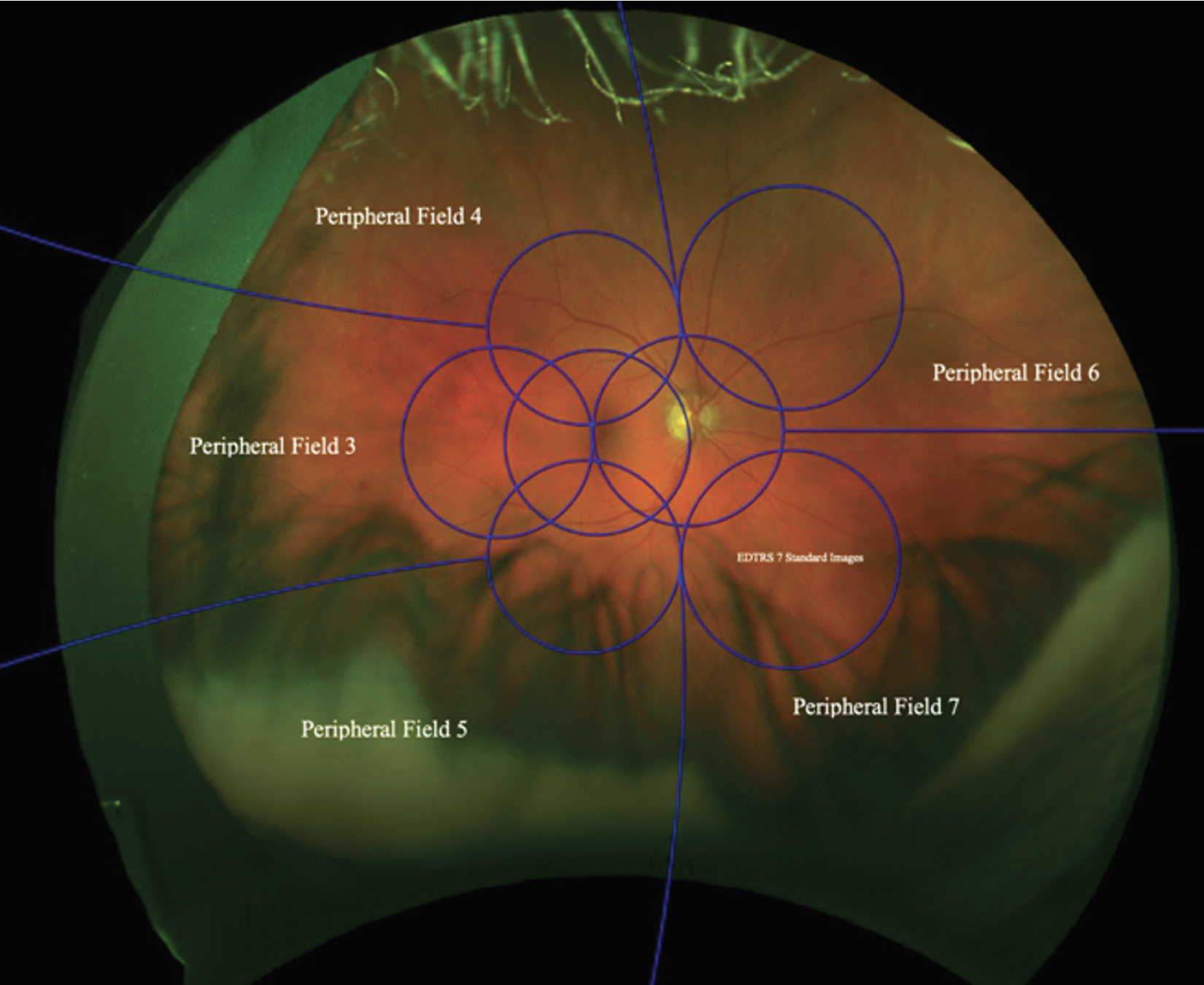 |
| Figure 1. Ultra-widefield color fundus photo of a right eye demonstrating the Early Treatment Diabetic Retinopathy Study seven-standard-field images (blue circles) and peripheral fields 3 to 7. (Courtesy DRCR Retina Network) |
Based on grading of the masked UWF color photos, 45, 40, 26 and 43 percent of eyes with baseline mild, moderate, moderately severe and severe NPDR, respectively, demonstrated a two-or-more step increase in DRSS over four years.
While DR progression wasn’t as expected based on baseline DRSS status as determined on UWF color photos, any DRSS worsening was seen in 31, 37, 43 and 56 percent of eyes with mild, moderate, moderately severe, and severe NPDR with baseline grading of ETDRS photographs.
DRCR Protocol AA found no significant relationship between the baseline presence of UWF color PPLs and two-or-more-step worsening in DRSS (38 percent of eyes with baseline color PPLs vs. 43 percent without). However, eyes with baseline UWF FA PPLs had a significantly greater risk of two-or-more-step worsening in DRSS (50 percent for eyes with baseline UWF FA PPLs vs. 31 percent without UWF FA PPLs).
The primary outcome of DRCR Protocol AA was to determine if PPLs predicted a two-or-more-step increase in the DRSS or required treatment for DR. Overall, the trial demonstrated that baseline UWF FA PPLs were associated with greater risk of DR worsening. These eyes were found to have a 70 percent greater risk of DR progression compared to eyes without UWF FA PPLs over four years. Baseline UWF color PPLs weren’t found to be predictive of DR worsening.6
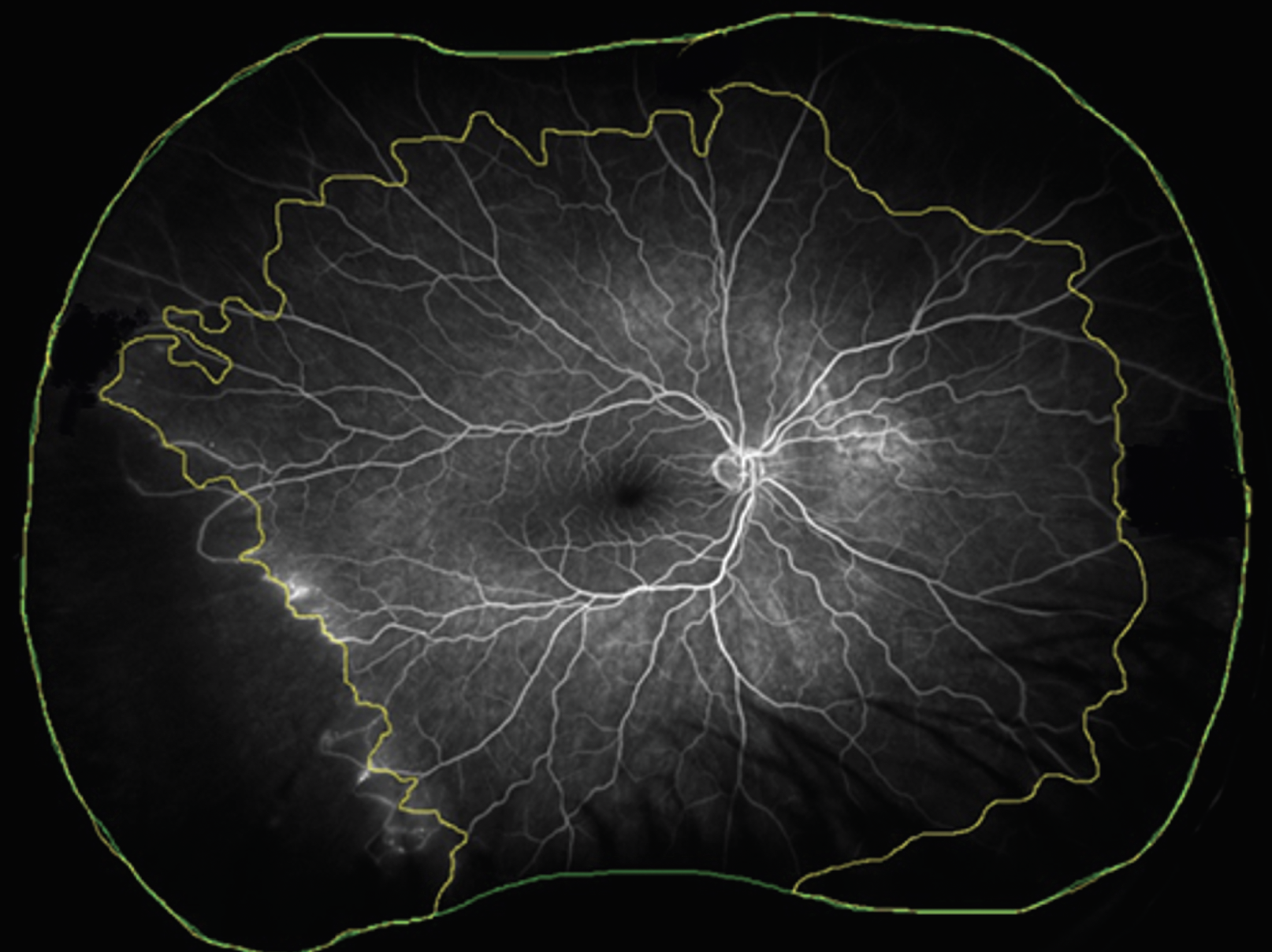 |
| Figure 2: Ultra-widefield fluorescein angiography demonstrating total gradable area (mm2, within the green line) and area of nonperfusion (mm2, between the yellow and green lines). Nonperfusion index is calculated as the ratio of the area of nonperfusion to the total gradable area. (Courtesy DRCR Retina Network) |
Retinal non-perfusion
DRCR Protocol AA also analyzed the retinal nonperfusion area (NPA) and nonperfusion index (NPI) measured on UWF FA (Figure 2). NPI was defined as NPA (mm2) divided by total gradable area (mm2). Of the 508 eyes with gradable baseline UWF FA nonperfusion, 9 percent had no nonperfusion.
Eyes with a greater area of nonperfusion were more likely to have type 1 diabetes, a longer duration of diabetes, higher baseline DRSS score and higher amounts of UWF FA PPLs.
Similar to the PPL analysis, the primary outcome of the NPA/NPI analysis was the proportion of eyes with two-or-more-step worsening in DRSS or required treatment for DR over four years. Twenty-six percent of eyes with no baseline nonperfusion met this primary outcome. However, 43, 38 and 46 percent of eyes in the low, medium and high nonperfusion subgroups, respectively, met the primary outcome.
Eyes with higher NPI were associated with a higher risk of progression to PDR. Other risk factors associated with higher rate of DR worsening were higher NPI in the posterior pole and midperiphery, ETDRS fields 6 and 7, and the superior, inferior and nasal extended periphery (Figure 2).14
Bottom line
Protocol AA provides us a foundation for using UWF color fundus photography and FA when evaluating eyes with NPDR. Over four years, PPLs on UWF FA and NPI can be used as markers to identify eyes that are more likely to have DR progression. While longer-term results are unknown, it’s likely that these markers portend worse DR prognosis at longer intervals as well.
These eyes should be monitored closely for evidence of progression and patients should be appropriately counseled. While historically FA wasn’t routinely used in eyes with NPDR, the use of UWF FA may help us better predict and counsel NPDR patients for DR progression and to determine appropriate monitoring intervals. RS
REFERENCES
1. No authors listed. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786-806.
2. Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785-791.
3. Gangaputra S, Almukhtar T, Glassman AR, et al. Comparison of film and digital fundus photographs in eyes of individuals with diabetes mellitus. Invest Ophthalmol Vis Sci. 2011;52:6168-6173.
4. Scott IU, Bressler NM, Bressler SB, et al. Agreement between clinician and reading center gradings of diabetic retinopathy severity level at baseline in a Phase 2 study of intravitreal bevacizumab for diabetic macular edema. Retina. 2008;28:36-40.
5. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3:843-849.
6. Marcus DM, Silva PS, Liu D, et al. Association of predominantly peripheral lesions on ultra-widefield imaging and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140:946-954.
7. Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65-73.
8. Ashraf M, Shokrollahi S, Salongcay RP, Aiello LP, Silva PS. Diabetic retinopathy and ultrawide field imaging. Semin Ophthalmol. 2020;35:56-65.
9. Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949-956.
10. Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154:549-559.e2.
11. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: Distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587-2595.
12. Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465-2472.
13. Talks SJ, Manjunath V, Steel DH, Peto T, Taylor R. New vessels detected on wide-field imaging compared to two-field and seven-field imaging: Implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 2015;99:1606-1609.



