 |
|
Bio Dr. Obeid is a first year clinical retina fellow at Wills Eye Hospital, Philadelphia. Dr. Hsu is an attending physician at Mid Atlantic Retina and the Retina Service of Wills Eye Hospital. |
Workup and imaging
Snellen visual acuity was 20/30 in the right eye and 20/70 in the left eye. Intraocular pressure was 20 and 16 mmHg in the right and left eye, respectively. Anterior segment evaluation of the right eye was within normal limits except for early nuclear sclerosis and a patent peripheral iridotomy. Anterior segment examination of the left eye revealed no injection of the conjunctiva/sclera, scattered fine keratic precipitates on the endothelium, a deep anterior chamber with trace cell, no frank iris nodules or posterior synechiae, and early nuclear sclerosis.
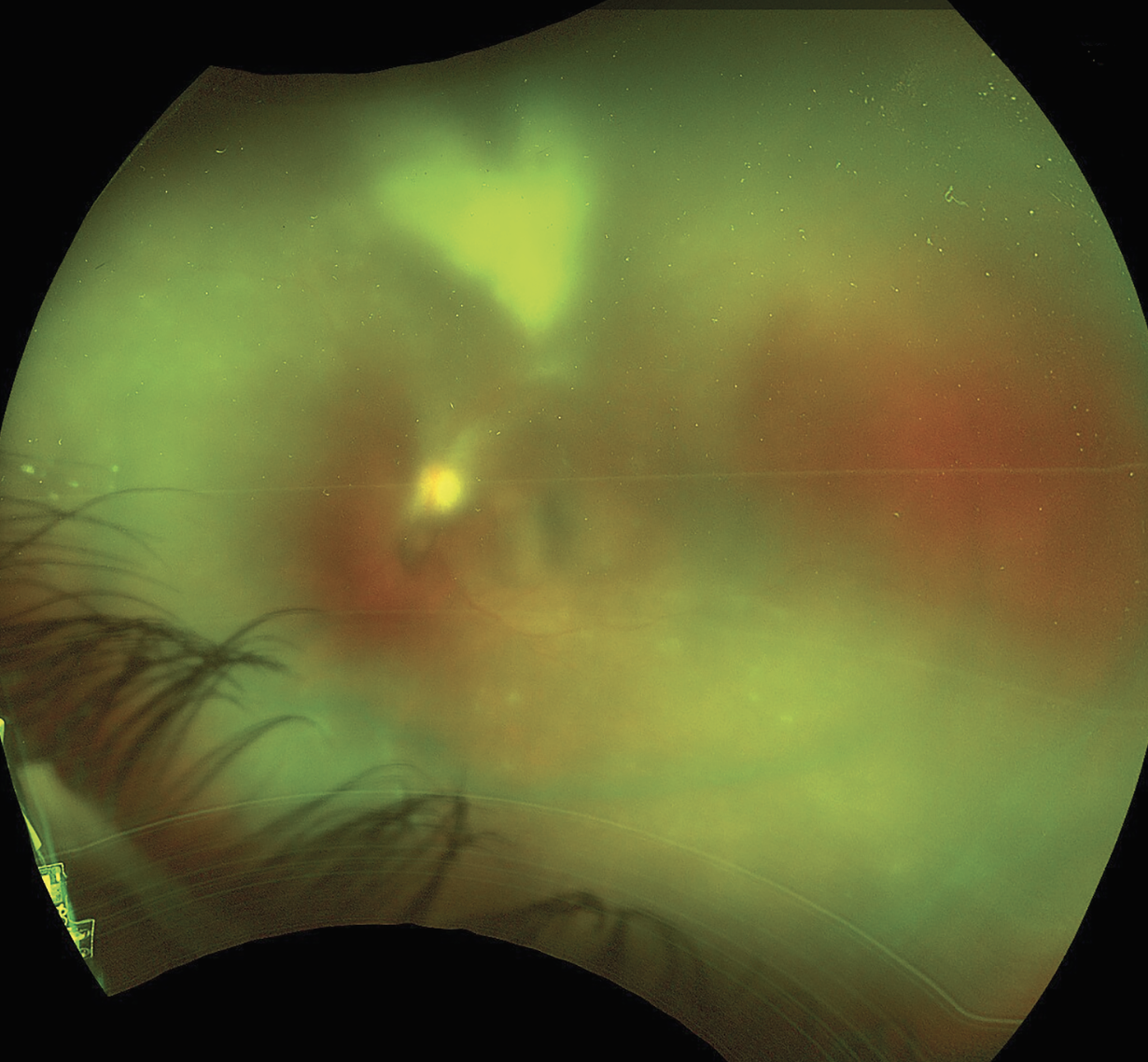
Dilated fundus exam of the right eye was normal. The dilated fundus exam of the left eye was notable for 2+ cells with 3+ vitreous haze. The nerve appeared flat with no frank disc edema. There was difficulty visualizing the retinal vessels and the macula. A large patch of retinal whitening, at least greater than three disc diameters in size, just sparing the macula was present. Abutting the inferior arcades were multiple white satellite lesions (Figure 1).
 |
|
Figure 1. Ultra-widefield fundus photo of the left eye demonstrating dense vitritis with a large patch of retinal whitening and multiple white satellite lesions. |
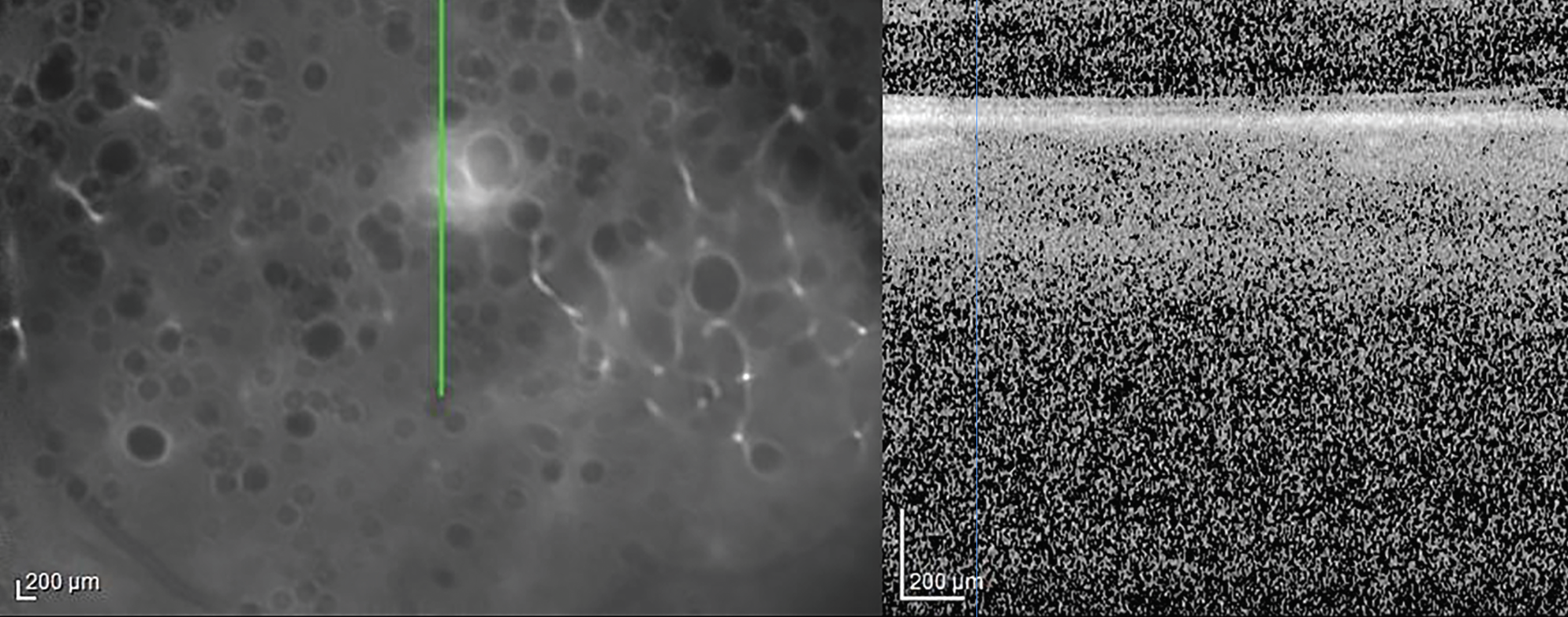
Fluorescein angiography of the right eye was relatively unremarkable. Fluorescein angiography of the left eye revealed early blockage and late staining of the area of retinitis with peripheral vascular leakage (Figure 2). Optical coherence tomography of the right eye revealed a clear hyaloid, intact retinal laminations and a normal choroid. OCT of the left eye was of poor signal owing to the dense vitritis, however, there did appear to be vitreous cell with a hyper-reflective foci overlying the internal limiting membrane (Figure 3). Grossly, the retinal laminations appeared to be intact with no frank evidence of intraretinal or subretinal fluid.
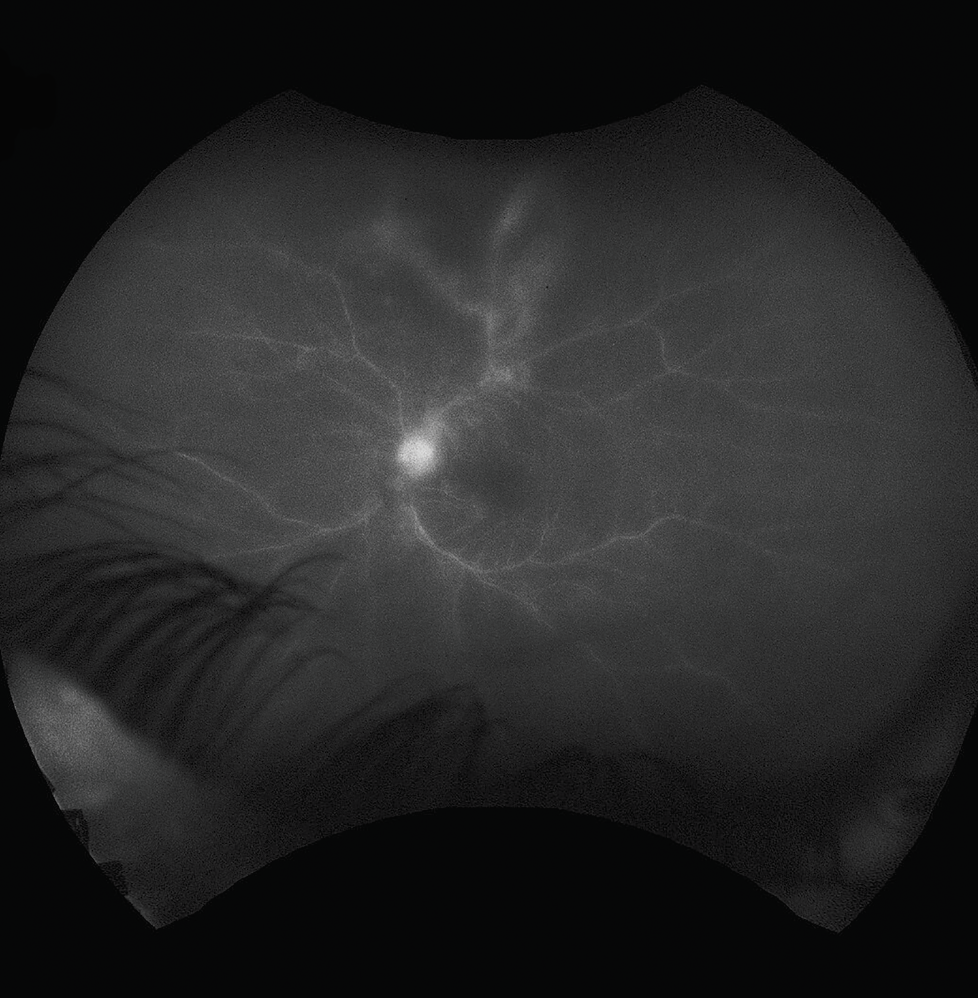 |
| Figure 2. Fluorescein angiography of the left eye demonstrating hyperfluorescence of the disc as well as blockage and staining by the area of retinitis with some leakage from adjacent vessels. |
Initial diagnosis
At this stage, the clinical picture was concerning for acute retinal necrosis secondary to a herpetic viral etiology. A diagnostic anterior chamber paracentesis was performed and an intravitreal injection of foscarnet was given in the left eye. A complete blood count, comprehensive metabolic profile, FTA-ABS/RPR, a QuantiFERON gold, angiotensin converting enzyme level, and a chest X-ray were ordered. The patient was initiated on Valtrex 1,000 mg /three times a day and instructed to follow up by the end of the week.
Polymerase chain reaction testing of the anterior chamber fluid revealed 10,500 copies/ml of toxoplasmosis but was negative for HSV and CMV. The diagnosis of atypical acute retinitis secondary to toxoplasmosis was made and the patient was started on Bactrim DS b.i.d. orally until follow-up. On further questioning, the patient revealed she had been diagnosed with HIV “years ago” but didn’t believe her initial diagnosis. She was instructed to follow up with an infectious disease specialist for testing along with a CD4 count. The patient followed up one week after initiating Bactrim DS. Her exam was notable for improvement in vitreous haze (1-2+), resolution of the satellite lesions and regression of the main superior lesion with borders now more clearly defined than in the prior fundus examination. She was continued on Bactrim DS with plans for close follow-up (Figure 4.)
 |
|
Figure 3. Optical coherence tomography of the left eye. There is a poor signal owing to the dense vitritis, however, there appears to be vitreous cell with a hyper-reflective foci overlying the internal limiting membrane. |
Discussion
Toxoplasmosis is caused by the obligate intracellular protozoan parasite, Toxoplasma gondii. Transmission of the protozoan can be secondary to direct exposure to cats or the consumption of undercooked meat. The original infection is often asymptomatic.1 Ocular toxoplasmosis is divided into congenital vs. postnatally acquired disease. If acquired while in utero, patients may have several systemic malformations, such as hydrocephalus, intracranial calcifications, myocarditis, hepatitis and cataracts.2
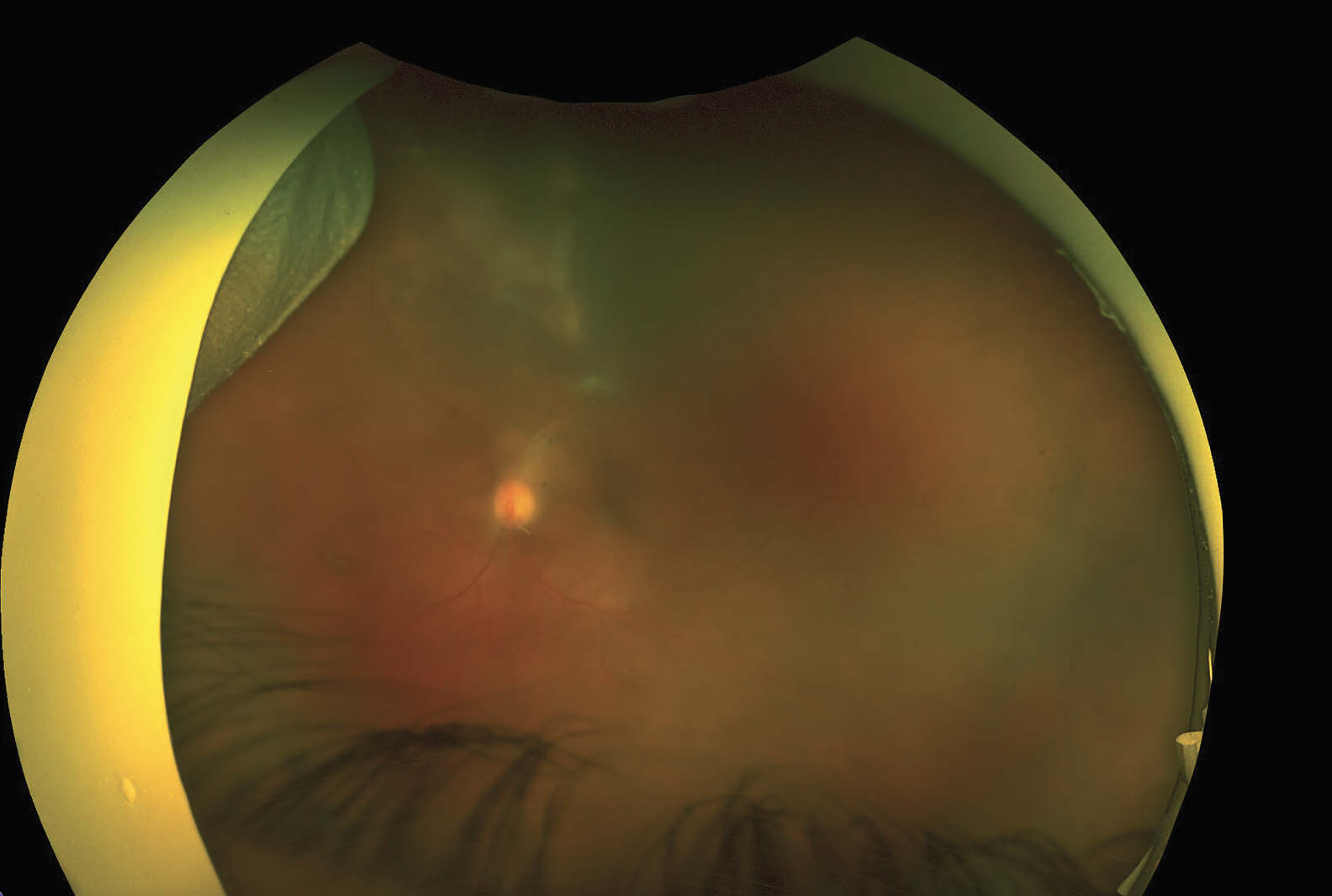 |
| Figure 4. Follow-up ultra-widefield fundus photo of the left eye one week after initiating systemic Bactrim DS. There’s improvement in vitreous haze (1-2+), resolution of the satellite lesions and regression of the main superior lesion with borders now more clearly defined than in the prior fundus photo. |
The classical presentation of ocular toxoplasmosis is a posterior uveitis with a unilateral chorioretinal lesion with associated vitritis, producing a “headlight in the fog” appearance. These lesions are often found proximal to an old chorioretinal scar, signaling reactivation secondary to rupture of intraretinal cysts which in turn stimulates a localized immune response.3 However, ocular toxoplasmosis can present in a variety of different manners which may obfuscate the clinical picture.4 This leads to delays in diagnosis and treatment.
One atypical presentation, seen more frequently in immunocompromised patients, is a severe aggressive retinochoroiditis.4 In these presentations, lesions are large, multiple and can be bilateral.4 Pre-existing retinal scars may be absent owing to the parasite either being newly acquired or disseminated.5 Satellite lesions may also be present. The area involved may progressively worsen rather than “burn out” and relapse on discontinuation of medication. In fact, some cases may even progress into a panophthalmitis.6 Additionally, these patients may have concomitant cerebral toxoplasmosis.7 Distinguishing such a presentation from other etiologies like cytomegalovirus retinitis may be difficult, although there are some clues which may increase suspicion for toxoplasmosis; these include a more prominent vitreous inflammation, a smoother edge with few satellite lesions, and a lack of intraretinal hemorrhages.3 There appears to be no reliable method to distinguish this presentation from acute retinal necrosis secondary to a herpetic virus.
In these situations, diagnostic testing becomes essential.8 Testing includes PCR, serology and immunohistochemical identification of the parasite.3 PCR testing of intraocular fluid is of great value, although the sensitivity with ocular fluid may be dependent on the PCR protocol. Sensitivity may also be dependent on immunocompetence, as PCR testing in immunocompetent patients is often less sensitive as a result of the robust immune response.9 Serological methods can also help determine if an infection was recently acquired or is chronic.
Imaging in ocular toxoplasmosis may also aid in the diagnosis and monitoring of the disease. OCT helps with monitoring the retinal lesions for resolution and scarring. Fluorescein angiography can be helpful in detecting small active lesions with hyperfluorescence emanating from the periphery and migrating toward the center.3 Additionally, it can detect segmental vasculitis and papillitis. Ultrasound can be useful in cases with dense vitritis and a poor view on dilated fundus exam. Ultimately, a combination of clinical presentation, diagnostic testing and multimodal imaging will help in raising suspicion and diagnosing such atypical ocular toxoplasmosis cases.3
There are no clear guidelines when it comes to the treatment of ocular toxoplasmosis. Small peripheral lesions in immunocompetent patients who aren’t very symptomatic could be observed closely without systemic therapy and are expected to improve over one to two months. Factors which may prompt the physician to treat include the patient’s immunocompetence, location of the lesion, visual acuity, involvement of the nerve and other clinical parameters.3 The classic therapy for ocular toxoplasmosis is a combination of pyrimethamine, sulfadiazine and prednisone (to be started 48 hours after initiation of antiparasitic agents). Initiation of prednisone may be omitted in immunocompromised patients.3 Alternative regimens to the pyrimethamine/sulfadiazine combination include trimethoprim-sulfamethoxazole and clindamycin. Another alternative involves an intravitreal injection of clindamycin with or without dexamethasone.3
Both intravitreal and systemic therapy seem to demonstrate equal efficacy, although there’s some evidence to suggest that patients who are IgM positive would benefit from systemic therapy and patients who are IgM negative would benefit from local therapy.10 The rationale is that IgM-positive patients have a systemic infection which is more readily targeted by systemic therapy. Benefits of local therapy include fewer side effects and the avoidance of major adverse effects encountered with systemic therapy.
In conclusion, although ocular toxoplasmosis often has a familiar appearance, some atypical presentations may cause a delay in diagnosis and treatment. Therefore, a clinician should always have a high index of suspicion and send for relevant testing to rule out this vision-threatening disease, particularly in susceptible populations. RS
REFERENCES
1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet Lond Engl. 2004;363: 9425 :1965-1976.
2. Maldonado YA, Read JS, Committee on Infectious Diseases . Diagnosis, treatment, and prevention of congenital toxoplasmosis in th e United States. Pediatrics. 2017;139: 2 :e20163860.
3. Kalogeropoulos D, Sakkas H, Mohammed B, et al. Ocular toxoplasmosis: A review of the current diagnostic and therapeutic approaches. Int Ophthalmol. 2022;42: 1 :295-321.
4. Smith JR, Cunningham ET. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol. 2002;13: 6 :387-392.
5. Johnson MW, Greven GM, Jaffe GJ, Sudhalkar H, Vine AK. Atypical, severe toxoplasmic retinochoroiditis in elderly patients. Ophthalmology. 1997;104 :1 :48-57. 6. Moorthy RS, Smith RE, Rao NA. Progressive ocular toxoplasmosis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;115 :6 :742-747.
7. Cochereau-Massin I, LeHoang P, Lautier-Frau M, et al. Ocular toxoplasmosis in human immunodeficiency virus-infected patients. Am J Ophthalmol. 1992;114 :2 :130-135.
8. Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111:4:716-725.
9. Bourdin C, Busse A, Kouamou E, et al. PCR-based detection of Toxoplasma gondii DNA in blood and ocular samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2014;52:11:3987-3991.
10. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118:1:134-141.




