 |
|
Bios Dr. Felfeli is an Dr. Mandelcorn is an associate professor of ophthalmology at the University of Toronto. DISCLOSURES: The authors have no relevant financial disclosures. |
In this past year, many refinements in pharmacological randomized clinical trials in retina have occurred, ranging from delivery modes to treatment regimens. Here, we summarize a selection of the latest published RCTs and ongoing protocols in our space (Table, below).
We identified these studies through a literature search for retinal clinical studies published in 2022 in the Cochrane and Medline databases. While this selection only captures some of the RCTs published in 2022, we selected them to represent a range of retinal conditions and therapies and to demonstrate research productivity across the world. We categorize them by disease entity.
Diabetic retinopathies
• Topical dexamethasone for diabetic macular edema. This multicenter, European Phase II study compared topical high-concentration dexamethasone ophthalmic suspension eye drops (OCS-01) with a matching vehicle eye drops in 144 patients.1
The study measured efficacy as change from baseline to Week 12 in Early Treatment Diabetic Retinopathy Study letter score and central macular thickness (CMT) in 144 randomized patients. Topical OCS-01 was more effective in improving CMT compared to the vehicle, and ETDRS letter score was better in eyes with lower baseline vision.
• Intravitreal faricimab for DME. The YOSEMITE and RHINE trials were novel Phase III clinical trials across 16 countries to evaluate intravitreal faricimab every eight weeks (q8w), per personalized treatment interval, compared to an aflibercept control group in optimizing patient outcomes in DME.2
The trials followed 1,891 patients every four weeks over one year. Faricimab demonstrated a similar profile to aflibercept for adverse events and intraocular inflammation. Faricimab also showed noninferiority in one-year ETDRS letter improvements and anatomical improvements compared to aflibercept. Moreover, the personalized treatment interval analyses demonstrated that faricimab may have dosing durability of up to 16 weeks, posing a potential area for further individualized therapy in clinical practice.
• Aflibercept for retinal nonperfusion in proliferative DR. In its second year, the RECOVERY trial evaluated the
impact of dosing frequency for intravitreal aflibercept injections on retinal nonperfusion after monthly dosed subjects crossed over to quarterly dosing and vice versa.3
Measuring total retinal nonperfusion area in 40 patients and 40 eyes, the study found a greater increase in retinal nonperfusion at one year in the group initially dosed quarterly. However, after crossover, both groups had an increase in retinal nonperfusion. The authors noted these findings have clinical implications for follow-up intervals, prognoses and the risk of further deterioration.
 |
• Highly concentrated docosahexaenoic acid supplementation in patients with nonproliferative DR. The PAOXRED study in Spain studied the effect of a docosahexaenoic acid (DHA) nutritional supplement compared to placebo on NPDR.4
Amongst the 170 randomized patients, the investigators measured changes in NPDR and HbA1C every six months. Neither arm demonstrated significant change in either measure at two years. However, the investigators concluded that further studies are needed to more effectively explore the efficacy of nutritional supplements in DR.
Retinal vein occlusion
• Acetazolamide/bevacizumab combination therapy vs. bevacizumab monotherapy. This Iran-based trial compared intravitreal bevacizumab (IVB) and oral acetazolamide combination therapy to IVB monotherapy in patients with macular edema secondary to RVO.5
This study of 54 eyes of 52 patients observed no additional short-term benefits for anatomical or functional outcomes as both regimens found a reduction in central macular thickness and an improvement in best-corrected visual acuity after three months. As such, oral acetazolamide adjuvant therapy may not have any additional therapeutic benefit in the short term.
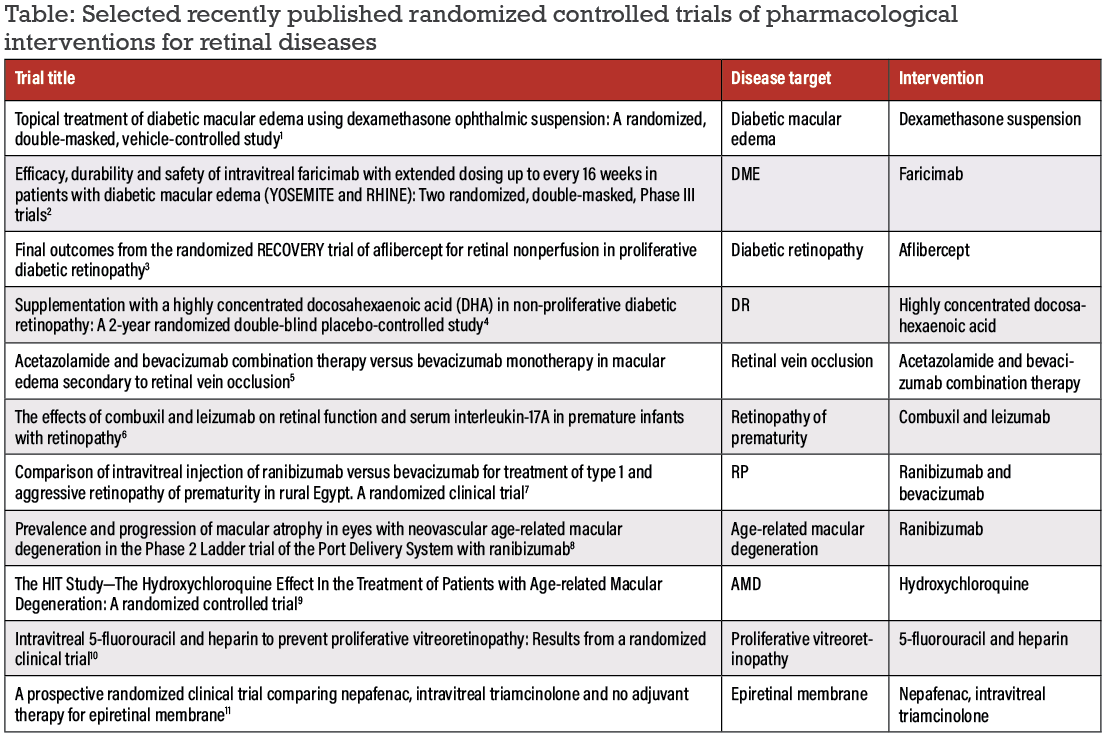 |
Retinopathy of prematurity
• Combuxil vs. leizumab on retinal function in premature infants. Investigators in China compared these two agents in treating ROP in 60 infants.6 The leizumab group was found to have reduced serum interluekin-17A levels and reduced intraocular pressure compared to the combuxil control group.
The patients treated with leizumab had a significantly lower incidence of complications, including corneal opacity, lens opacity, preretinal and vitreous hemorrhage, endophthalmitis and traction retinal detachment. The investigators concluded that, clinically, leizumab is an effective treatment for ROP and further studies can help to verify and hone this form of treatment.
• Ranibizumab vs. bevacizumab. This study in rural Egypt of 36 eyes of 18 infants with aggressive ROP compared the efficacy of monotherapy with these two agents.7 The authors found that initial regression of ROP occurred at a higher rate in the ranibizumab group, but not significantly.
The ranibizumab-treated eyes also had a higher incidence of late recurrence, which wasn’t significant. As such, both monotherapies appear to be effective for regressing ROP, and any differences may not be clinically significant based on results from this study.
Age-related macular degeneration
• Port Delivery System with ranibizumab. A preplanned exploratory endpoint of the Phase II Ladder trial, first reported in 2019, examined the Port
Delivery System (PDS) with ranibizumab for neovascular AMD. Ladder compared the prevalence and progression of macular atrophy with continuous ranibizumab treatment with PDS with treatment via a bolus intravitreal injection. The trial enrolled 220 eyes.8
The rates of macular atrophy and area of macular atrophy lesions throughout the Ladder trial were similar across all treatment arms, thus this exploration found no evidence of worse macular atrophy with PDS compared to bolus monthly intravitreal injections. Larger trials focusing on macular atrophy can confirm this finding because previous trials have indicated variable growth rates of macular atrophy and anti-VEGF treatment.
• The HIT study of hydroxychloroquine. The HIT study, conducted in
Israel, investigated a potential protective effect of hydroxychloroquine (HCQ) against the development of nAMD.9 The study compared visual acuity, CMT and drusen number and size in 110 patients receiving HCQ vs. placebo. The study authors reported reduced visual deterioration, and drusen growth and formation among the treated patients. As such, hydroxychloroquine could be a preventative treatment to change the course of AMD.
 |
Perioperative medical management
• Intravitreal 5-fluorouracil and heparin to prevent proliferative vitreoretinopathy. This trial aimed to evaluate the effect of intraoperative adjuvant therapy with 5-fluorouracil and low-molecular weight heparin compared to standard care for prevention of PVR.10
This study enrolled 325 patients with primary rhegmatogenous retinal detachment considered to be at high-risk for PVR. The key measurement was the
development of grade CP 1 or higher PVR within 12 weeks. However, the rate of PVR didn’t differ between the therapies and placebo, indicating that the search to find an agent that reduces PVR continues.
• Nepafenac vs. triamcinolone and no adjuvant therapy for epiretinal membrane. Our group conducted this multicenter, Canada-based trial evaluating efficacy of topical nepafenac vs. intravitreal triamcinolone acetonide at the conclusion of pars plana vitrectomy for epiretinal membrane.11 We randomized 80 patients and obtained measurements before surgery and one and two months postoperatively.
The study found no advantage for either therapy compared to no adjuvant therapy. The nepafenac group did demonstrate greater reduction in macular thickness after the operation and better recovery in BCVA, albeit neither was statistically significant. This finding suggests that
anti-inflammatory agents provide no benefit in terms of postoperative macular morphology after vitrectomy surgery.11
Ongoing multicenter clinical trials
Exciting studies evaluating new indications and novel treatments for existing therapies include the following.
• A Phase III study of anti-VEGF treatment for prevention of vision-threatening DR in high-risk eyes. Completed in May 2022, this study recruited 399 U.S. patients to evaluated the efficacy and safety of intravitreal aflibercept injections compared to sham injections for prevention of PDR or center-involved DME (NCT02634333).12 Hoping to compare long-term visual outcomes in eyes that receive anti-VEGF therapy early in the disease course compared to treatment with vision loss, the study may provide insight into better outcomes for patients with vision-threatening complications of diabetes.
• VITAL trial of vitamin D3 or omega-3 fatty acids in AMD. VITAL stands for VITamin D and OmegA-3 TriaL, which is an ongoing RCT evaluating these two daily dietary supplements and the risk of developing cancer, heart disease and stroke without prior history (NCT01169259).13
The ancillary study will examine whether these supplements, compared to placebo, will reduce the incidence and/or progression of AMD. With a sample size of 25,875 patients, this multicenter study is estimated to be completed in 2024. This may provide us further insight into the benefits of supplements.
• Fenofibrate for prevention of DR worsening. A Phase III RCT is comparing fenofibrate, a oral fibrate that targets blood lipid levels, with placebo for slowing DR progression in patients with NPDR (NCT04661358).14
Started in 2021 and scheduled for completion in 2027, this study also aims to determine the feasibility of retina specialists collaborating with internists and/or endocrinologists to prescribe and monitor fenofibrate. This will hopefully inspire a new strategy to prevent vision complications from diabetes.
• Bevacizumab for ROP. Another study to be on the lookout for is the Bevacizumab Treatment For Posterior Zone I Retinopathy of Prematurity trial in Canada and the United States (NCT04634578).15 Started last year with completion scheduled for next year, this study will evaluate whether low-dose bevacizumab will be successful in severe cases of ROP compared with the standard dose in approximately 80 infants.
With these findings, we can aim to evaluate the safety and efficacy of the lower dose and possibly enhance treatment outcomes for patients with type 1 ROP.
Bottom line
We’ve seen considerable growth in the development of new modes of delivery, testing of novel medications and the refinement of our current regimens in medical retina over the past year. These RCTs, among many others, demonstrate how the field of retina continues to home in on treatment delivery and efficacy by personalizing treatment and follow-up intervals, adding adjuvant therapies and exploring nutritional supplements. With many new RCTs on the horizon, we’re sure to expect more advancements in the treatment of retinal disease in the coming year. RS
REFERENCES
1. Stefansson E, Loftsson T, Larsen M, et al. Topical treatment of diabetic macular edema using dexamethasone ophthalmic suspension: A randomized, double-masked, vehicle-controlled study. DX-211 study group, editor. Acta Ophthalmologica. 2023;101:22–33.
2. Wykoff CC, Abreu F, Adamis AP, et al, YOSEMITE and RHINE Investigators. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741–755.
3. Wykoff CC, Nittala MG, Villanueva et al. Final outcomes from the randomized RECOVERY trial of aflibercept for retinal nonperfusion in proliferative diabetic retinopathy. Ophthalmology Retina. 2022;6:557–566.
4. Piñas García P, Hernández Martínez FJ, Aznárez López N, Castillón Torre L, Tena Sempere ME. Supplementation with a highly concentrated docosahexaenoic acid (DHA) in non-proliferative diabetic retinopathy: A 2-year randomized double-blind placebo-controlled study. Antioxidants. 2022;11:116.
5. Karimi S, Nikkhah H, Nafisi H, et al. Acetazolamide and bevacizumab combination therapy versus bevacizumab monotherapy in macular edema secondary to retinal vein occlusion. J Francais d’ophtalmologie. 2023;46:322–326.
6. Ren H, Su G, Xu S, Zhou L, Cai S. The effects of combuxil and leizumab on retinal function and serum interleukin-17a in premature infants with retinopathy. Computational Math Methods Med. 2022;2022:6371994.
7. Tawfik GM, Shahein EA, Dabour SA, Hassanein D, Elshewy AM. Comparison of intravitreal injection of ranibizumab versus bevacizumab for treatment of type 1 and aggressive retinopathy of prematurity in rural Egypt. A randomized clinical trial. BMJ Open Ophthalmology. 2022;7:e001173.
8. Jaffe GJ, Cameron B, Kardatzke D, Ives J, Barteselli G, Gune S. Prevalence and progression of macular atrophy in eyes with neovascular age-related macular degeneration in the phase 2 ladder trial of the port delivery system with ranibizumab. Ophthalmology. Retina. 2022;6:786–795.
9. Yahalomi T, Pikkel Y, Arnon R, Porat D, Pikkel J. The HIT study—the Hydroxychloroquine Effect in the Treatment of Patients with Age-related Macular Degeneration: A randomized controlled trial. Medicina (Kaunas). 2023;59:551.
10. Schaub F, Schiller P, Hoerster R, et al. Intravitreal 5-fluorouracil and heparin to prevent proliferative vitreoretinopathy: Results from a randomized clinical trial. Ophthalmology. 2022;129:1129–1141.
11. Mandelcorn ED, Al-Falah M, Zhao LD, Kertes P, Devenyi R, Lam W-C. A prospective randomized clinical trial comparing nepafenac, intravitreal triamcinolone and no adjuvant therapy for epiretinal membrane. Acta Ophthalmologica. 2022;100:e297–e303.
12. ClinicalTrials.gov. Anti-VEGF treatment for prevention of PDR/DME. NCT identifier 02634333. Available at: https://clinicaltrials.gov/study/NCT02634333. Accessed November 28, 2023.
13. ClinicalTrials.gov. Age-related Macular Degeneration (AMD) in the Vitamin D and Omega-3 Trial (VITAL). NCT identifier 01782352. Available at: https://clinicaltrials.gov/study/NCT01782352. Accessed November 28, 2023.
14. ClinicalTrials.gov. Fenofibrate for prevention of DR worsening. NCT identifier 04661358. Available at: https://clinicaltrials.gov/study/NCT04661358. Accessed November 28, 2023.
15. ClinicalTrials.gov. Bevacizumab treatment for type 1 ROP. NCT identifier 04634578. Available at: https://clinicaltrials.gov/study/NCT04634578. Accessed November 28, 2023.




