Take-home points
|
 |
Bios Dr. Villafuerte-Trisolini is a vitreoretinal specialist from Peru and postdoctoral fellow at the University of California Davis in Sacramento. Dr. Yiu is a professor of ophthalmology at the UC Davis, where he’s a vitreoretinal surgeon and clinician-scientist. DISCLOSURES: Dr. Villafuerte- Dr. Yiu is a consultant to AbbVie, Adverum, Alimera Sciences, Bausch + Lomb, Boehringer Ingelheim, Clearside Biomedical, Endogena Therapeutics, Genentech/Roche, Gyroscope, Intergalactic, Iridex, Janssen, Myrobalan, NGM Biopharmaceuticals, Novartis, Ray Therapeutics, Regeneron, RegenXBio, Stealth Therapeutics, Thea, Topcon and Zeiss. |
Recent advances have led to the development of novel techniques for delivering drugs to the posterior pole of the eye. One such technique gaining momentum is suprachoroidal injection using microneedles.1–3 Suprachoroidal injections involve delivery into the potential space between the choroid and sclera. This technique offers several advantages over other methods.
Potential advantages of suprachoroidal administration
The suprachoroidal space brings the injectate into close proximity with the choroid, retinal pigment epithelium and retina, which can provide higher bioavailability to the diseased tissues while minimizing impact on anterior segment tissues.1–5 Thus, suprachoroidal injections of a steroid suspension could provide lower risk for ocular hypertension or cataract formation.
Another advantage is the minimally invasive nature of the suprachoroidal injection, which allows it to be performed in an office-based setting with relative ease compared to other methods, such as suprachoroidal catheterization which requires more invasive surgical techniques.4
What makes the microneedles different
Microneedles are hollow-bore needles that are just slightly longer than the thickness of the sclera and conjunctiva, typically around 900 μm. The product developers specifically chose this length to reach the suprachoroidal space reliably.6 The first approved use of microneedles in the suprachoroidal space was for the administration of a triamcinolone acetonide suspension via suprachoroidal injection (Xipere, Clearside Biomedical/Bausch + Lomb) for the treatment of uveitic cystoid macular edema.7
Across clinical trials, the 900-μm microneedle can successfully access the suprachoroidal space on the first injection attempt in 78 percent of patients without the need to measure the scleral thickness.8 For the remaining patients, a second attempt can be made in a different quadrant of the globe or with using a slightly longer 1,100-μm needle.
Although suprachoroidal microneedles are only currently approved for the treatment of uveitic CME, their use has been evaluated for retinal vein occlusions and diabetic macular edema,2,5,9,10 and is also undergoing trials for the delivery of tyrosine kinase inhibitors (TKIs) for neovascular age-related macular degeneration.
These microneedles are also under investigation as mode of delivering viral vectors for gene therapy, suspensions, hydrogels and sustained-release microspheres.11–13 Therefore, learning how to perform the suprachoroidal injection technique will be an important addition to the retinal specialist’s repertoire of skills.
 |
 |
Patient considerations
Choosing the right patients for suprachoroidal injections is critical to ensure a good outcome, especially when the physician is still in the early stages of learning this technique. For example, although scleral thickness doesn’t have be measured before the injection, patients with known scleral thinning, high myopia or axial elongation may not be the best initial candidates.
Additionally, patients with a history of glaucoma, hypotony, a previous trabeculectomy or glaucoma shunt, or recent cataract or retinal surgery, especially involving scleral buckling, may have higher risks for complications. Although these aren’t absolute contraindications, many weren’t included in clinical trials using suprachoroidal microneedles, so the risk profile in real-world use is currently unknown.
Managing patient expectations
Because the suprachoroidal injection technique is very different from intravitreal injections, managing patient expectations is absolutely critical, especially for those who have received intravitreal injections previously.
Patients should be aware that the injection will take more time, require careful positioning and could cause some discomfort. Most patients report a sensation of a “pressure wave” or headache similar to the “brain freeze” from eating ice cream. A very slow speed of injection can mitigate this. Patients should also be told that there may be a need to exchange needles to find the optimal length for that patient.
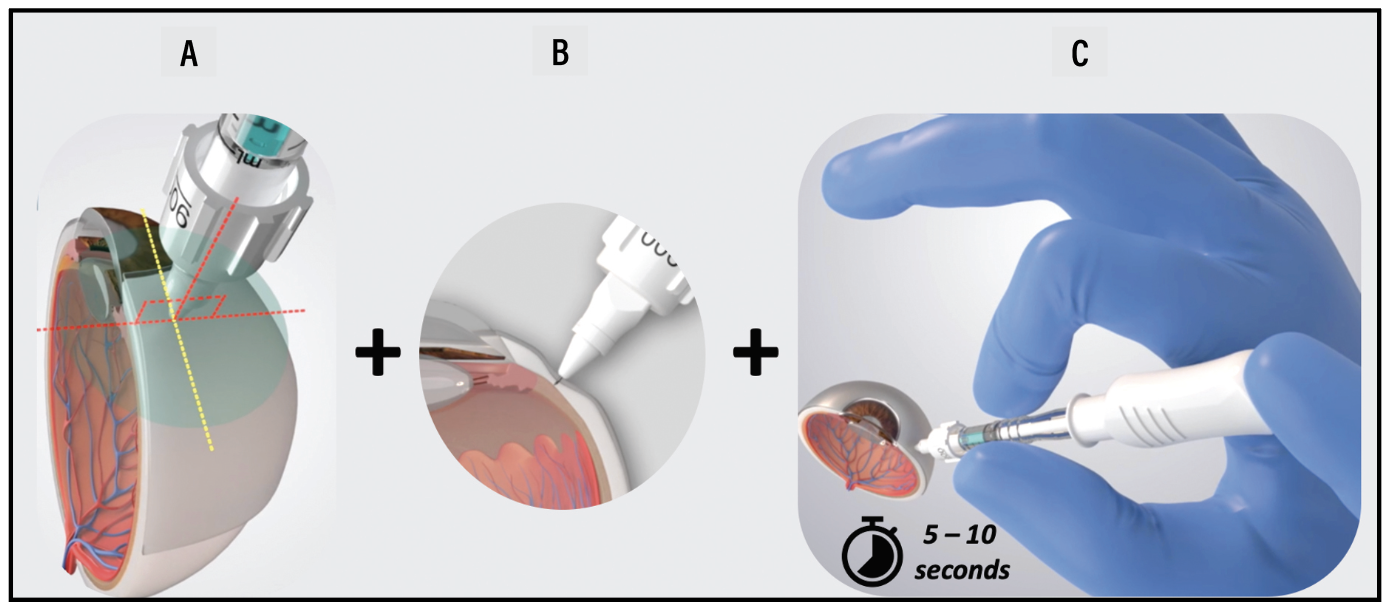 |
| The appropriate injection technique for suprachoroidal space (SCS) injection with the SCS Microinjector includes the following steps: A) Maintaining perpendicular alignment of the device relative to the injection location; B) While, at the same time, pressing against the surface of the eye with the needle hub to create a dimple, a localized depression; and C) Once loss of resistance is observed, inject slowly, over 5 to 10 seconds. (Courtesy Bausch + Lomb) |
Strategies for success
Preinjection pupil dilation can aid in monitoring the eye after the injection. Pupil dilation isn’t essential; it depends on the physician’s preference.
In clinical trials, suprachoroidal injection success rate in the superotemporal quadrant was 78 percent compared to 65 percent for the inferotemporal quadrant, so the superotemporal quadrant is slightly preferred.8
Often, simply applying slightly firmer pressure and ensuring perpendicular orientation will be sufficient to achieve a successful injection. If you use the 1,100-μm microneedle, fill the syringe with more injectate and prime the microneedle to minimize air bubbles or underdosing. Repeat injection site prep when switching between quadrants or needles to ensure sterility.
Postinjection care and monitoring
Postinjection monitoring, such as checking intraocular pressure, should be similar to other standard postinjection protocols. Same-day bilateral suprachoroidal injections may be appropriate, depending on the physician’s judgment and the patient’s condition. Each eye should be treated as a separate procedure with different vials of medication and different injecting devices.
Inform caregivers of common side effects, such as subconjunctival hemorrhage, as well as the signs and symptoms of more serious potential complications, such as infection, retinal detachment or suprachoroidal hemorrhage. Although rare, patients and their caregivers should be aware of them and seek medical attention if concerning symptoms arise.
Potential complications
No cases of endophthalmitis were reported across eight Phase II and III studies of suprachoroidal triamcinolone. This isn’t unexpected, because the risk of endophthalmitis in the absence of perforation into the vitreous cavity should be very low. Given the mechanism of action for suprachoroidal injections, physicians may want to watch for signs of scleritis or periocular inflammation instead.
Although most clinical trials using suprachoroidal triamcinolone showed low rates of cataract and IOP elevation, many of these studies may have been too short in duration to observe these complications. Close monitoring and awareness remains important until more long-term,
real-world data become available.1,7,9,14,15
Bottom line
Suprachoroidal injections are a new and innovative technique that may provide improved bioavailability to the posterior pole while minimizing exposure and adverse effects on the anterior segment. Despite the ease of this in- office procedure, the suprachoroidal injection technique requires setting patient expectations, maintaining perpendicular orientation and firm pressure with the microinjector, injecting gently and slowly, and preparing for the need to switch location or needle. Potential applications beyond delivering steroids, including angiogenesis inhibitors and gene therapies, may broaden the scope of suprachoroidal delivery in ophthalmic practice. RS
REFERENCES
1. Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58–66.
2. Campochiaro PA, Wykoff CC, Brown DM, et al. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: Results of the Tanzanite study. Ophthalmol Retina. 2018;2:320–328.
3. Chen M, Li X, Liu J, Han Y, Cheng L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release Off J Control Release Soc. 2015;203:109–117.
4. Hancock SE, Wan CR, Fisher NE, Andino RV, Ciulla TA. Biomechanics of suprachoroidal drug delivery: From benchtop to clinical investigation in ocular therapies. Expert Opin Drug Deliv. 2021;18:777–788.
5. Wykoff CC, Avery RL, Barakat MR, et al. Suprachoroidal space injection technique: Expert Panel Guidance.
6. Patel SR, Lin ASP, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011;28:166–176.
7. Fung S, Syed YY. Suprachoroidal space triamcinolone acetonide: A review in uveitic macular edema. Drugs. 2022;82:1403–1410.
8. Wan C rei, Kapik B, Wykoff CC, et al. Clinical characterization of suprachoroidal injection procedure utilizing a microinjector across three retinal disorders. Transl Vis Sci Technol. 2020;9:27.
9. Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD. Suprachoroidal corticosteroid administration: A novel route for local treatment of noninfectious uveitis. Transl Vis Sci Technol. 2016;5:14.
10. Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis. Ophthalmology. 2020;127:948–955.
11. Hackett SF, Fu J, Kim YC, et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials. 2020;243:119935.
12. Kansara V, Muya L, Wan C rei, Ciulla TA. Suprachoroidal delivery of viral and nonviral gene therapy for retinal diseases. J Ocul Pharmacol Ther. 2020;36:384–392.
13. Jung JH, Kim SS, Chung H, Hejri A, Prausnitz MR. Six-month sustained delivery of anti-VEGF from in-situ forming hydrogel in the suprachoroidal space. J Control Release Off J Control Release Soc. 2022;352:472–484.
14. Naftali Ben Haim L, Moisseiev E. Drug delivery via the suprachoroidal space for the treatment of retinal diseases. Pharmaceutics. 2021;13:967.
15. Ciulla T, Yeh S. Microinjection via the suprachoroidal space: a review of a novel mode of administration. Am J Manag Care. 2022;28(Suppl):S243–252.



