Innovations in Retina Surgery
Take-home points
|
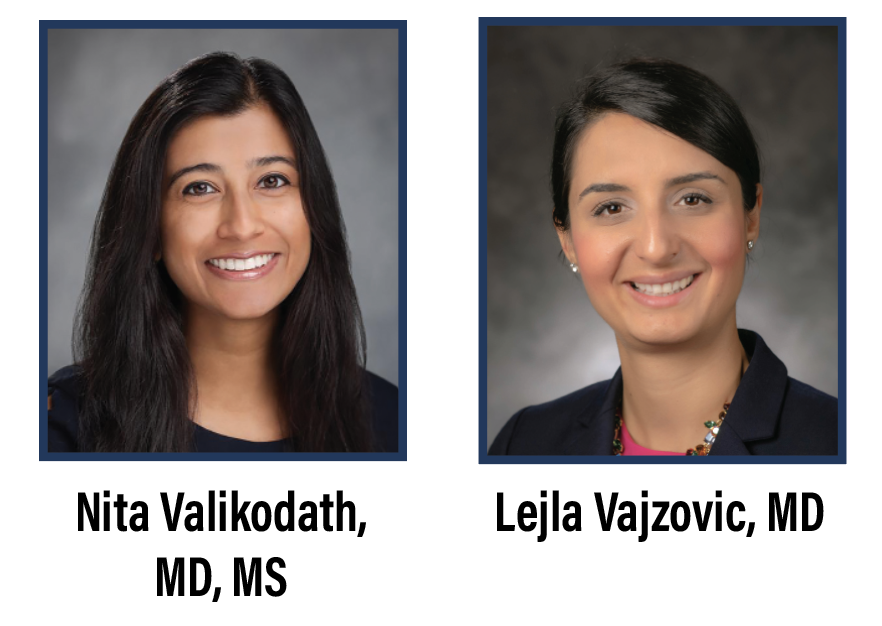 |
|
Bios Dr. Valikodath is a second-year vitreoretinal surgery fellow at Duke University, Durham, N.C., with a clinical research interest in imaging advancements in pediatric and adult retina. Dr. Vajzovic is an associate professor of ophthalmology specializing in adult and pediatric retinal diseases and surgery at Duke University. She has a clinical research emphasis in vitreoretinal surgical advancements, including heads-up display technology. DISCLOSURES: Dr. Valikodath has no relevant relationships to disclose. Dr. Vajzovic disclosed relationships with AGTC Corp., Alcon, |
Three-dimensional heads-up display is an exciting and innovative advancement in vitreoretinal surgery. The 3D system gives the surgeon a digital, stereoscopic, high-definition view of the surgical field on an external monitor without looking through the operating microscope.
Initially introduced by Claus Eckartdt, MD, the uses and indications for 3D heads-up display, or HUD, continue to expand.1–3 Through the use of a high-dynamic range camera, an image is projected onto a 55-inch organic light-emitting diode, high-definition display.4
Today, two 3D HUD models are available commercially in the United States: the Ngenuity 3D system (Alcon Laboratories) and the Artevo 800 system (Zeiss). Another form of heads-up display, the Beyeonics One system (Beyeonics Vision) uses a digital microscope that transmits a signal to a visor the surgeon wears. We will report on our experience with 3D HUD based on our use of the Ngenuity system.
Enhanced visualization
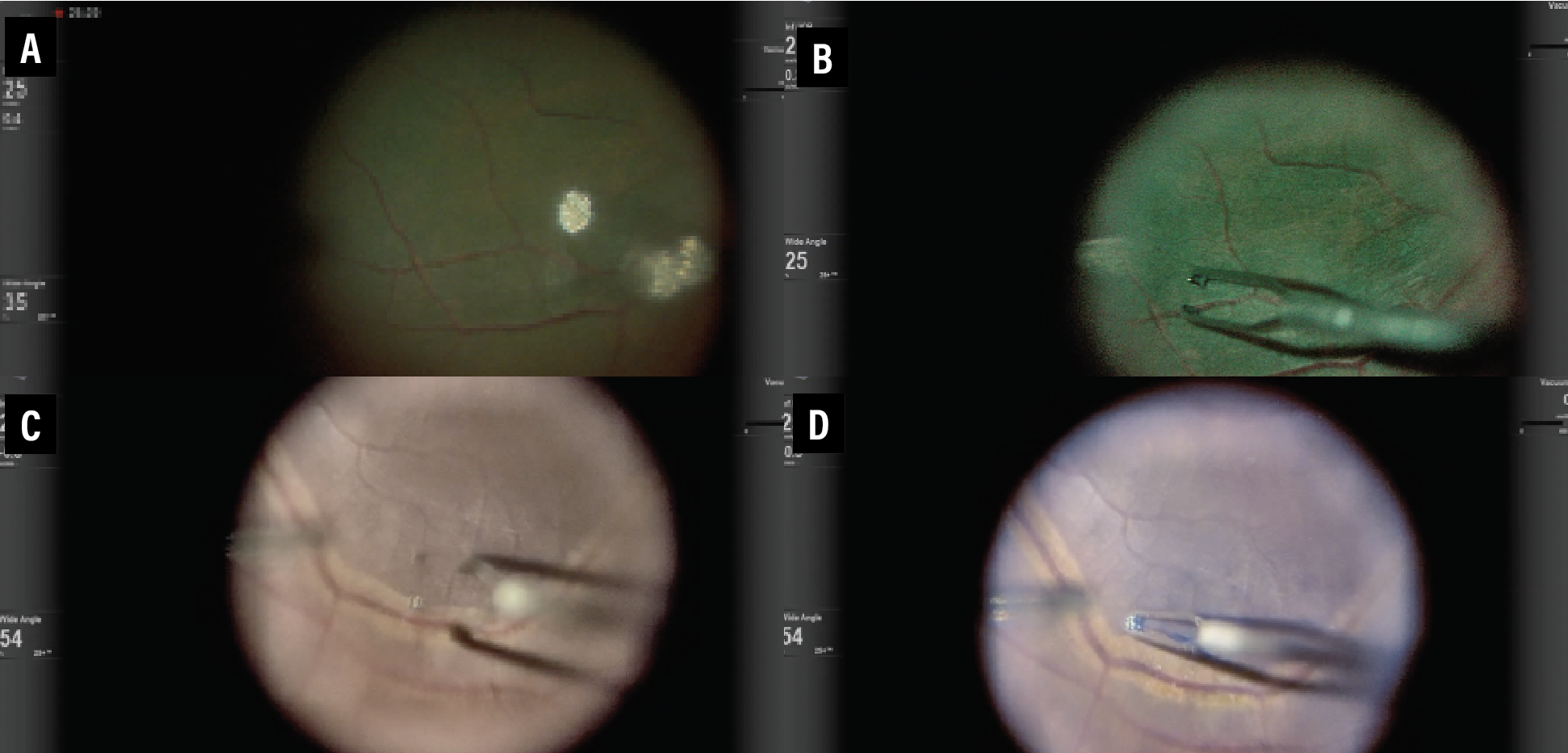
One of the main advantages of 3D HUD display includes enhanced high-resolution visualization and video chromatography. Various color profiles and camera gain adjustments can help digitally augment images to highlight pathology or areas of interest. This is especially useful to enhance visualization of vital dyes such as indocyanine green or Brilliant Blue G (Figure 1). The green boost and blue boost allow improved visualization of the internal limiting membrane after staining with ICG and BBG, respectively. Various color, contrast and tissue detail modes enable better visualization of the vitreous (Figure 2). Operators can customize the colored channels.
Other advantages of 3D HUD include improved stereopsis and depth of field compared with the standard operating microscope. The surgeon and viewers must wear passive polarized glasses to obtain stereopsis. The system can preserve magnification even with a larger field of view.2,5 Endoillumination settings are also adjustable and overall the system requires lower endoillumination (3 to 10 percent vs. 30 to 40 percent in the operating microscope), which is advantageous for avoiding phototoxicity.6,7
 |
| Figure 1. Comparison of standard (A and C), green boost (B) and blue boost (D) views during internal limiting membrane peeling for macular hole surgery. |
Shared surgical experience
The 3D glasses provide all OR surgical staff with a shared view of the surgery. This can be advantageous for OR flow as the surgical assistant, scrub technician and others anticipate progress and tools needed in surgery. This technology provides an important pedagogical value, especially in settings with trainees. The enhanced visualization allows for an improved learning experience and doesn’t depend on accommodative ability, which can be limited with the standard operating microscope.8,9 Also, 3D HUD systems can record and save videos.
Ergonomics
Surgeon ergonomics are important and can affect surgical performance. The height of the 3D HUD can be adjusted and the display can be positioned in a place that’s most comfortable for the surgeon. It eliminates the need to look through a microscope which can cause back and neck issues.
A high proportion of ophthalmologists with musculoskeletal disorders has been reported. Claus Eckardt, MD, and Eric Paulo, MD, reported that 91.7 percent of participants (n=20) preferred the ergonomics of the HUD over the traditional approach.3
A Saudi Arabia study (n=132) reported higher pain intensity in surgeons who used a standard operating microscope vs. HUD users.10 The study noted that HUD could help improve career longevity and productivity, but the monitor must be positioned appropriately at the beginning of the case or the surgeon may find themselves looking around the operating scope to see the display.
 |
| Figure 2. Three-dimensional heads-up display permits improved visualization of the vitreous through various color, contrast and tissue detail modes. The colored channels can be customized for each surgeon. |
Imaging
HUD can allow side-by-side or superimposed images of intraoperative optical coherence tomography images in real-time. The DISCOVER study, which evaluated microscope-integrated OCT with 3D visualization, reported its use in procedures such as macular surgery and retinal detachment repair with proliferative vitreoretinopathy.11
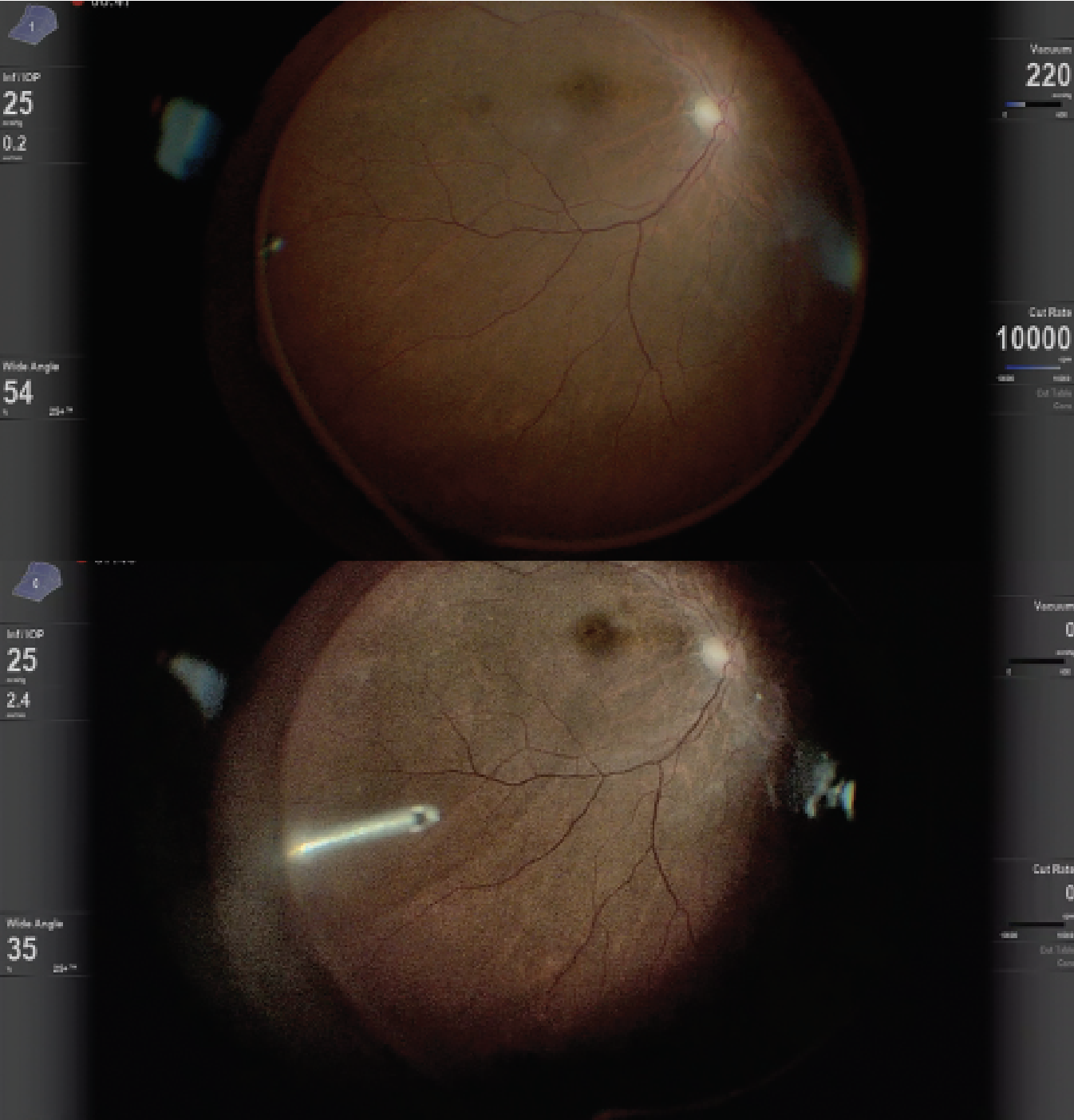
The surgeon can view the OCT image without looking away from the monitor. 3D HUD integrated with iOCT has also been investigated for gene therapy and subretinal tPA injection, and has aided in visualization of the retinal architecture to ensure proper delivery of subretinal drugs (Figure 3).12–17
 |
| Figure 3. Heads-up display during gene therapy placement with intraocular optical coherence tomography. |
Potential drawbacks
Using HUD technology involves a learning curve. Some surgeons have reported a lag between instrument movement and the display image. Other disadvantages include longer set-up and operating times for early users, physical space limitations and cost.
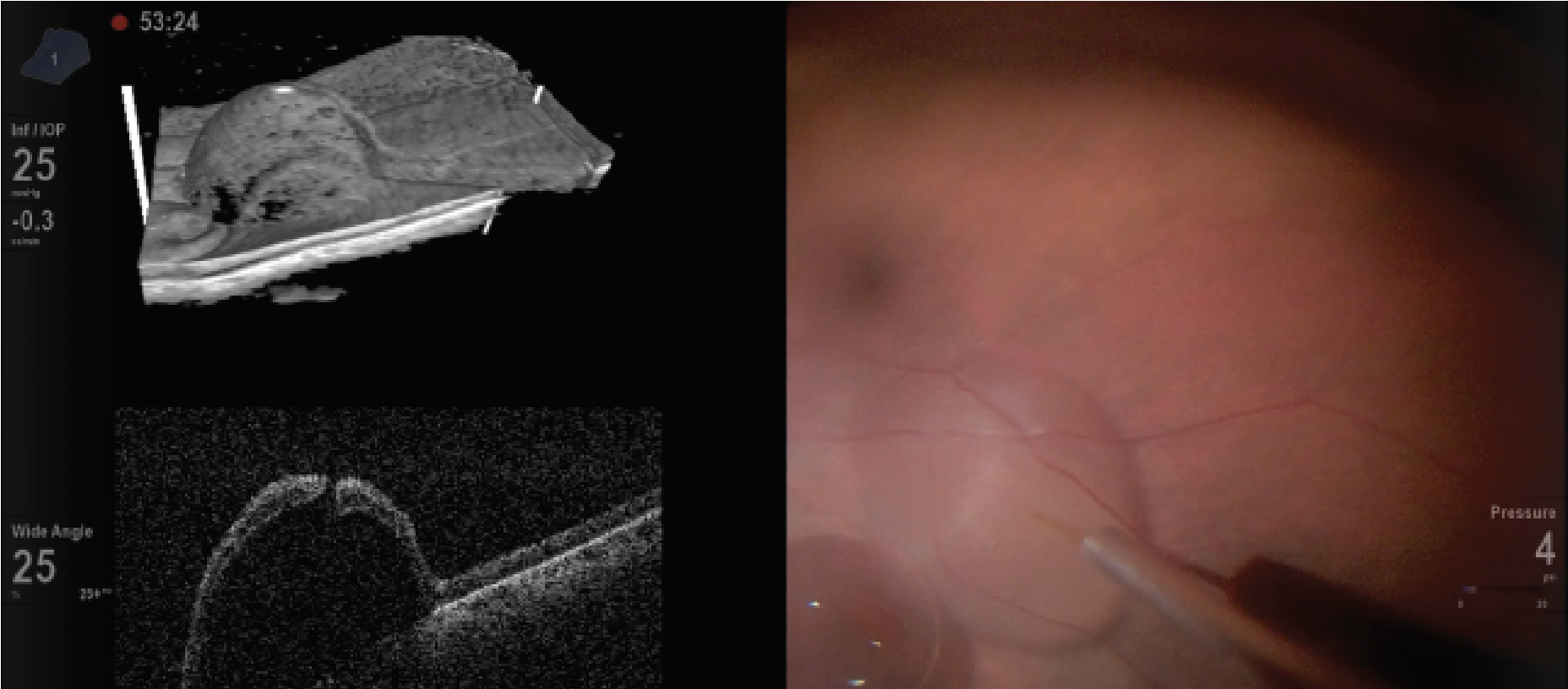
Ideally, the spatial layout should be coordinated with the OR team and anesthesia to position the monitor 1.5 meters from the surgeon, typically at the foot of the operating table (Figure 4). Lastly, one must view in 3D for the entire case. That may cause eye strain and asthenopia.
Some steps of vitreoretinal surgery may be more difficult with 3D HUD compared with the operating microscope. New users should start with straightforward cases, such as nonclearing vitreous hemorrhage or macular cases, instead of secondary intraocular lens or complex retinal detachments.
Endolaser can be more challenging with the 3D HUD because the laser beam and laser uptake can be more difficult to visualize. In one survey, fellows and residents reported worse experience with endolaser and closing compared with the standard operating microscope.9 However, these outcomes may also be affected by the initial learning curve and user experience.
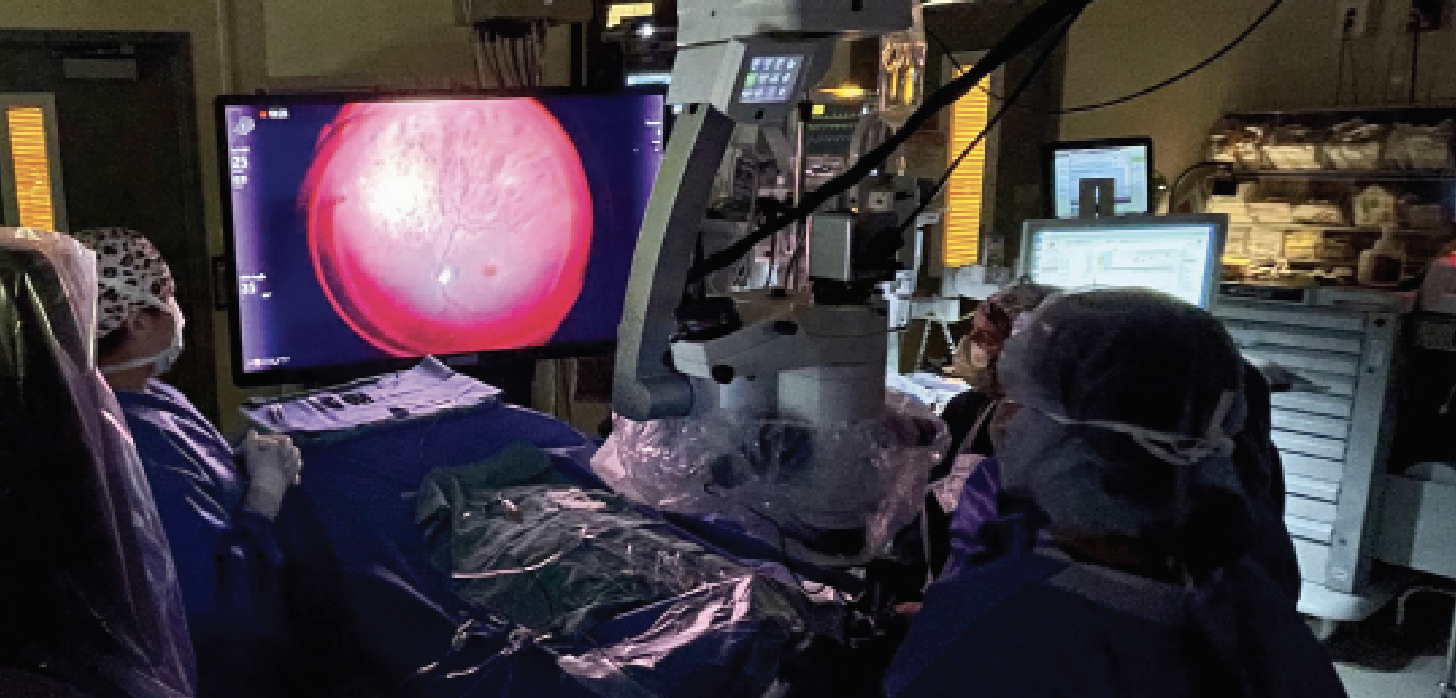 |
| Figure 4. Layout of the three-dimensional, heads-up display system in the operating room. |
Potential applications of 3D HUD
• Macular surgery. For epiretinal membrane peel and macular hole surgeries,
various stains, including BBG and ICG, can be used. The color filters of the HUD system can help to minimize or avoid the use of dyes. For example, the digital red-free filter can highlight ILM. Thomas Aaberg Jr., MD, of Retina Specialists of Michigan, has reported using half the concentration of the vital dye without affecting performance.
A Wills Eye Hospital study compared 3D HUD and the standard operating microscope for ERM and macular hole surgery.2 It showed that 3D HUD was safe for macular surgery, used lower-light settings and had similar outcomes compared to the conventional system. However, in this study, operating times were longer and surgeons found the operating microscope easier to use.2
An Italian study reported ease of use was higher in the 3D HUD group than the operating microscope group.18 These researchers found that surgeon satisfaction and ease of use with the 3D HUD system likely correlated to experience and differences in learning curve. Interestingly, another study evaluated VR fellows and surgical trainees performing macular hole closure, rates of which were higher in the 3D HUD group. The authors hypothesize that better visualization and instruction from mentors was possible with the 3D HUD system.19
• Retinal detachment and vitreous hemorrhage. Another study from Italy reported on 3D HUD for rhegmatogenous retinal detachment surgery with vitrectomy, finding that the 3D HUD group didn’t need triamcinolone while the standard microscope group did.20 The authors thought this was due to high-resolution images and use of digital filters with the 3D HUD system. They also reported reduced endoillumination settings in the 3D HUD group.
They found that the 3D HUD group had good outcomes with no redetachments and no major postoperative complications. Specific contrast adjustments can enhance vitreous visualization, leading to efficient removal of the vitreous hemorrhage and peripheral vitreous.
• Gene therapy. 3D HUD can be valuable when performing subretinal gene therapy. The enhanced depth of view and high-resolution display can allow for improved surgical performance especially with subretinal bleb creation and propagation. In addition, as we mentioned, incorporating iOCT on the same display offers advantages. Delivering the gene therapy subretinally is important, but reflux from the retinotomy site or leakage into the intravitreal cavity can occur. This can be subtle in the microscope view, but on 3D HUD the leakage can be seen on the display, often more evident from the shadow of the needle.
• Other uses. HUD has been used for more challenging cases such as pathologic myopic foveoschisis, dislocated intraocular lens removal and exchange, retinal prosthesis implantation, such as the Argus II device, and more.21–23
 |
Future directions
Artificial intelligence and computer vision can be incorporated into 3D HUD and enhance audiovisual feedback to the surgeon. In cataract surgery, a deep-learning algorithm was developed that could provide real-time identification of surgical phase.
In addition, AI could provide guidance during surgery by outlining a template for capsulorrhexis or displaying excessive turbulence.24 In vitreoretinal surgery, an AI algorithm helped localize tissues, such as the fovea, and instruments, such as the vitrector tool tip. This type of surgical guidance could potentially avoid unintended contact of important structures leading to safer surgery.25
Bottom line
3D HUD enhances visualization during vitreoretinal surgery and offers an advantage for surgical teaching through a shared surgical experience, although it involves a learning curve. RS
REFERENCES
1. Williams GA. Surgical viewing: Do you see what I see? Retina. 2017;37:1219.
2. Talcott KE, Adam MK, Sioufi K, et al. Comparison of a three-dimensional heads-up display surgical platform with a standard operating microscope for macular surgery. Ophthalmol Retina. 2019;3:244-251.
3. Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures: An experimental and clinical study. Retina. 2016;36:137-147.
4. González-Saldivar G, Chow DR. Optimizing visual performance with digitally assisted vitreoretinal surgery. Ophthalmic Surg Lasers Imaging Retina. 2020;51:S15-S21.
5. Freeman WR, Chen KC, Ho J, et al. Resolution, depth of field, and physician satisfaction during digitally assisted vitreoretinal surgery. Retina. 2019;39:1768-1771.
6. .Thornton S, Adam MK, Ho AC, Hsu J. Endoillumination levels and display luminous emittance during three-dimensional heads-up vitreoretinal surgery. Poster presented at: Annual Meeting of the Association for Research in Vision and Ophthalmology; May 4, 2016; Seattle, WA.
7. Adam MK, Thornton S, Regillo CD, Park C, Ho AC, Hsu J. minimal endoilluminat zion levels and display luminous emittance during three-dimensional heads-up vitreoretinal surgery. Retina. 2017;37:1746-1749.
8. Agranat JS, Miller JB. 3D surgical viewing systems in vitreoretinal surgery. Int Ophthalmol Clin. 2020;60:17-23.
9. Shoshany TN, Agranat JS, Armstrong G, Miller JB. The user experience on a 3-dimensional heads-up display for vitreoretinal surgery across all members of the health care team: A survey of medical students, residents, fellows, attending surgeons, nurses, and anesthesiologists. J Vitreoretin Dis. 2020;4:459-466.
10. Bin Helayel H, Al-Mazidi S, AlAkeely A. Can the three-dimensional heads-up display improve ergonomics, surgical performance, and ophthalmology training compared to conventional microscopy? Clin Ophthalmol. 2021;15:679-686.
11. Ehlers JP, Uchida A, Srivastava SK. The integrative surgical theater: combining intraoperative optical coherence tomography and 3D digital visualization for vitreoretinal surgery in the DISCOVER study. Retina. 2018;38:S88-S96.
12. Ehlers JP, Petkovsek DS, Yuan A, Singh RP, Srivastava SK. Intrasurgical assessment of subretinal tpa injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46:327-332.
13. Raynor W, Li JD, Sastry A, et al. Optimizing subretinal gene therapy delivery with microscope-integrated optical coherence tomography and novel suprachoroidal delivery system. Invest Ophthalmol Vis Sci. 2020;61:4487.
14. Hsu ST, Gabr H, Viehland C, et al. Volumetric measurement of subretinal blebs using microscope-integrated optical coherence tomography. Transl Vis Sci Technol. 2018;7:19.
15. Sastry A, Li JD, Raynor W, et al. Microscope-integrated OCT-guided volumetric measurements of subretinal blebs created by a suprachoroidal approach. Transl Vis Sci Technol. 2021;10:24.
16. Vajzovic L, Sleiman K, Viehland C, et al. Four-dimensional microscope-integrated optical coherence tomography guidance in a model eye subretinal surgery. Retina. 2019;39:S194-S198.
17. Li JD, Raynor W, Dhalla AH, et al. Quantitative measurements of intraocular structures and microinjection bleb volumes using intraoperative optical coherence tomography. Biomed Opt Express. 2023;14:352-366.
18. Romano MR, Cennamo G, Comune C, et al. Evaluation of 3D heads-up vitrectomy: Outcomes of psychometric skills testing and surgeon satisfaction. Eye (Lond). 2018;32:1093-1098.
19. Reddy S, Mallikarjun K, Mohamed A, et al. Comparing clinical outcomes of macular hole surgeries performed by trainee surgeons using a 3D heads-up display viewing system versus a standard operating microscope. Int Ophthalmol. 2021;41:2649-2655.
20. Coppola M, La Spina C, Rabiolo A, Querques G, Bandello F. Heads-up 3D vision system for retinal detachment surgery. Int J Retina Vitr. 2017;3:46.
21. Rachitskaya A, Lane L, Ehlers J, DeBenedictis M, Yuan A. Argus II retinal prosthesis implantation using three-dimensional visualization system. Retina. 2019;39:S199-S200.
22. Kantor P, Matonti F, Varenne F, et al. Use of the heads-up NGENUITY 3D Visualization System for vitreoretinal surgery: A retrospective evaluation of outcomes in a French tertiary center. Sci Rep. 2021;11:10031.
23. Zhang G, Yang M, Zeng J, et al. Comparison of intravitreal injection of ranibizumab versus laser therapy for zone II treatment-requiring retinopathy of prematurity. Retina. 2017;37:710-717.
24. Garcia Nespolo R, Yi D, Cole E, Valikodath N, Luciano C, Leiderman YI. Evaluation of Artificial intelligence-based intraoperative guidance tools for phacoemulsification cataract surgery. JAMA Ophthalmol. 2022;140:170-177.
25. Nespolo RG, Yi D, Cole E, Wang D, Warren A, Leiderman YI. Feature tracking and segmentation in real time via deep learning in vitreoretinal surgery: a platform for artificial intelligence-mediated surgical guidance. Ophthalmol Retina. 2023;7:236-242.



