|
 |
 |
After diabetic retinopathy, retinal vein occlusion is the second most common retinal vascular disease and carries a significant risk of vision loss, particularly when untreated, due to associated sequelae including macular edema, retinal ischemia and neovascularization. In the majority of cases, first-line treatment for macular edema is intravitreal injection of anti-VEGF. In some eyes, intravitreal corticosteroid is subsequently incorporated.
However, RVO-associated macular edema often recurs when the pharmacologic agent has worn off, requiring repeated and often monthly injections for disease control. In the last two decades, several surgical techniques have been described for the management of eyes with RVO, typically targeting the anatomic sites implicated in the pathoetiology of RVO, but they have largely fallen out of favor.
This article reviews variable study results of six different surgical techniques for treating RVO and provides insight into the few cases for which surgery may be considered.
Vitrectomy with arteriovenous sheathotomy
Since venous compression at the site of an arteriovenous crossing may predispose to branch retinal vein occlusion, surgical management has been geared toward this site of pathology. Pars plana vitrectomy with arteriovenous (AV) sheathotomy was first described in 1988 and again in 1999.1,2
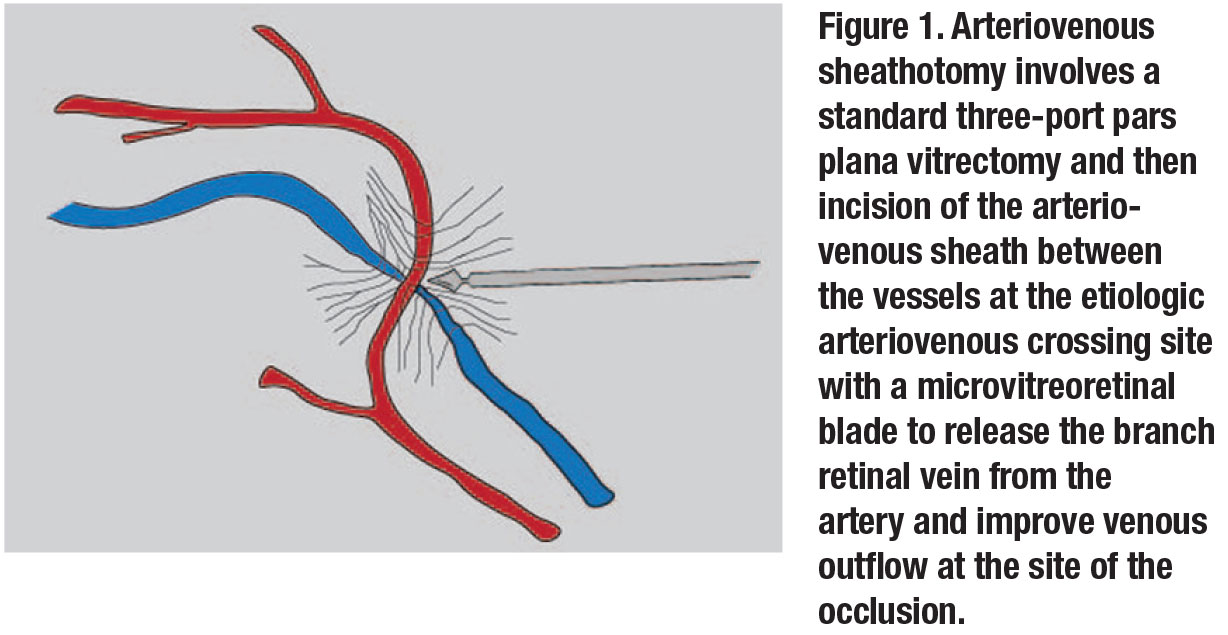
With this technique, a standard three-port PPV is performed followed by the use of a microvitreoretinal blade to incise and dissect open the AV sheath between the vessels at the etiologic arteriovenous crossing to release the branch retinal vein from the artery to improve venous outflow at the site of the occlusion (Figure 1). Fluorescein angiography is often used preoperatively to identify the best AV site to target.
While initial studies of this technique were promising, further studies showed that even though up to one-third of eyes had resolution of macular edema and some visual improvement in some series,3,4 complications were not uncommon. They included localized retinal detachment at the site of the sheathotomy.3 In most cases, venous outflow didn’t improve and visual results were lackluster. Given the lack of reproducible substantial visual acuity gains, AV sheathotomy is rarely performed in 2020.
 |
Vitrectomy with radial optic neurotomy
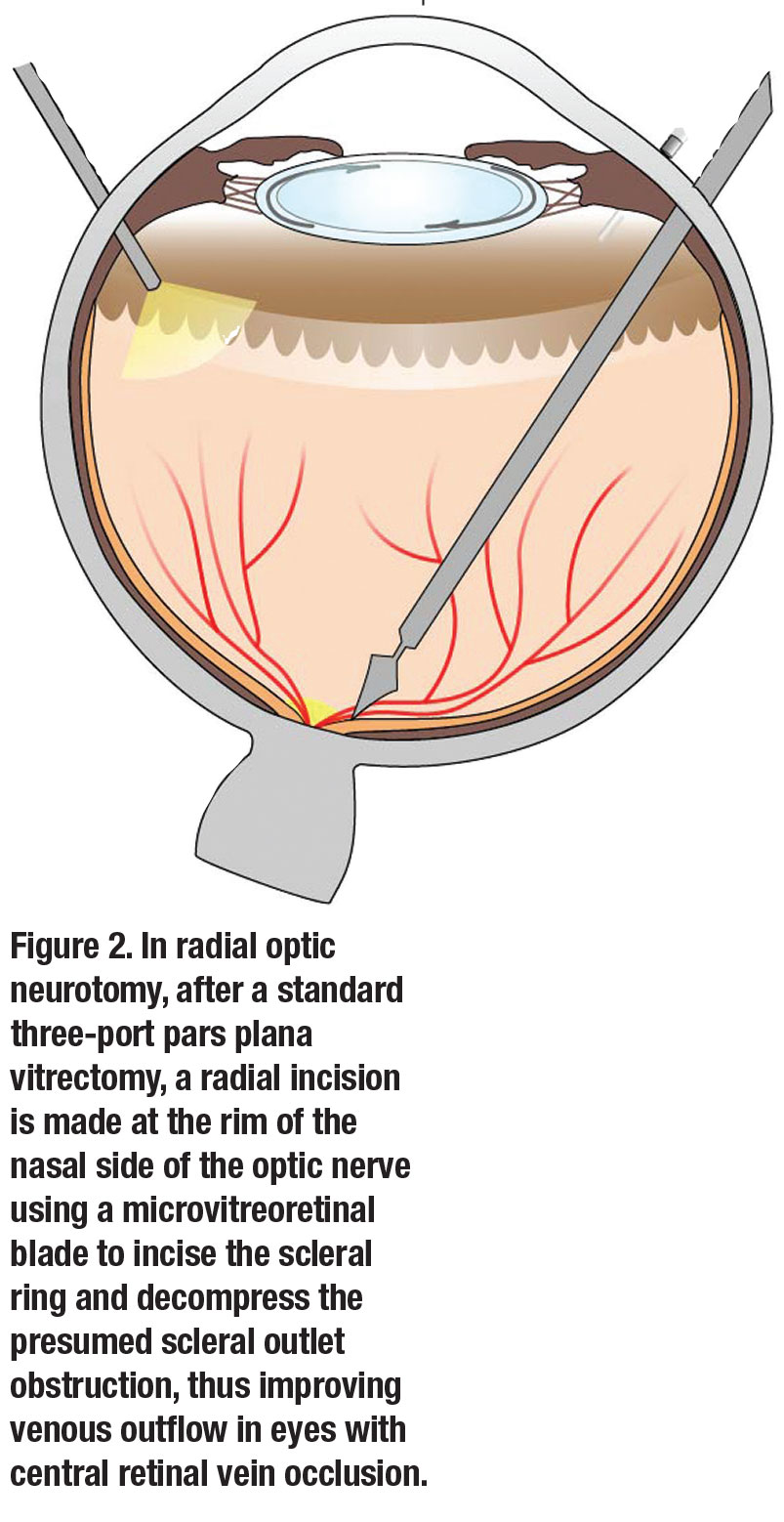
In eyes with central retinal vein occlusion, many authors have described a type of “compartment syndrome” at the scleral outlet. They proposed that relieving this outlet obstruction by performing vitrectomy with radial optic neurotomy (RON) could improve venous outflow in these eyes.5-7
This approach involves performing a standard three-port PPV and then making a radial incision at the rim of the nasal side of the optic nerve to incise the scleral ring and decompress the presumed scleral outlet obstruction, thus improving venous outflow in the central retinal vein (Figure 2, page 30).
Some of these studies reported significant improvement in visual acuity.5-7 However, most eyes that underwent RON did not have improved vision. Additionally, adverse events including choroidal neovascularization at the RON site have been reported.6 It’s also unclear if vitrectomy with removal of the posterior hyaloid alone leads to resolution of the macular edema and improved visual outcomes in these cases, rather than the effects of the RON itself.5
 |
Chorioretinal anastomosis with or without vitrectomy
A third surgical technique that has been described to improve retinal perfusion through direct anatomic intervention in eyes with both CRVO and BRVO is the creation of a chorioretinal anastomosis. The thought is that making a connection between a retinal vein and the choroidal venous circulation may create a venous detour so that venous outflow can bypass the occluded retinal vein.
While this could be performed in the clinic with application of laser pulses to rupture the wall of a branch vein and adjacent Bruch membrane to promote anastomosis formation during the healing process, a chorioretinal anastomosis can also be created during standard three-port PPV and posterior hyaloid detachment using transvitreal venipuncture.8
However, the side effects of this technique are potentially significant. They include preretinal fibrosis, segmental retinal ischemia and choroidovitreal neovascularization, so this surgical technique has not been widely adopted. If creation of a chorioretinal anastomosis is sought in select eyes, laser in the clinic is often the method of choice to do so.
Vitrectomy with retinal vein cannulation
Surgical retinal vein cannulation during vitrectomy surgery in eyes with CRVO has also been explored with promising improvements in visual acuity.9 Because histopathologic studies have demonstrated a thrombus at the level of the lamina cribrosa in eyes with CRVO, targeted retinal venous cannulation can allow direct access to the thrombus, which may be displaced with injection of balanced salt solution alone or dissolved with recombinant tissue plasminogen activator, ocriplasmin (Jetrea, Oxurion) or other thrombolytic.
Animal studies are evaluating retinal cannulation for the treatment of RVO, both unassisted and with robotic assistance for more distal, difficult-to-access vasculature.10,11 Recently, a report of four RVO patients undergoing robot-assisted retinal vein cannulation was published, showing both technical feasibility and a reasonable safety profile.12
 |
Vitrectomy with and without ILM peeling
Prior studies have evaluated the role of vitrectomy with removal of the posterior hyaloid with and without internal limiting membrane peeling to treat macular
edema associated with RVO, particularly when the edema is refractory to treatment with intravitreal
anti-VEGF or corticosteroid therapy.
Multiple studies have shown decreased retinal thickness and improved visual acuity in eyes with RVO after PPV with removal of the posterior hyaloid alone, without ILM peeling.13-15 Prior studies have suggested that the vitreous may be a reservoir for inflammatory and growth factor mediators, including interleukin-6 and vascular endothelial growth factor.16-18 Therefore, removal of the vitreous alone may lead to resolution of macular edema on a cellular level with reduced retinal hypoxia, decreased vascular permeability, and improved oxygenation of the inner retina as more oxygenated aqueous fills the vitrectomized vitreous cavity.19
In one study, eyes with higher preoperative intravitreal anti-VEGF levels had less visual acuity gains after PPV.15 This suggests that there may be a correlation and the potential to predict which eyes with RVO will be most responsive to surgical management in the future.
While ILM peeling is thought to primarily relieve mechanical forces contributing to macular edema, such as tangential traction, the added step of ILM peeling may provide the extra benefit of decompression of the inner retina.20 Furthermore, removal of the ILM may also possibly decrease macular edema by stimulating a neural repair process.21 While ILM peeling may confer anatomic benefits, whether or it provides a functional benefit is still up for debate.
The largest study of this approach, the European VitreoRetinal Society Macular Edema Study, evaluated visual acuity outcomes in a nonrandomized fashion at 24 months postoperatively following PPV with ILM peeling vs. treatment with intravitreal anti-VEGF or steroid injections. In more than 700 cases of CRVO or BRVO, the study reported significantly greater visual gains in eyes treated with surgical management.22
On the other hand, smaller retrospective case series have reported a decrease in retinal thickness without significant visual acuity gains in eyes with CRVO after PPV with ILM peeling.23,24 These studies have shown heterogeneity in the time duration from development of RVO to surgery, extent of retinal ischemia, lens status and surgical technique. No randomized trial has evaluated the utility of PPV with or without ILM peeling for the treatment of macular edema secondary to RVO.
Pitfalls and potential of vitrectomy surgery in eyes with RVO
Reperfusion of the retina continues to be a primary goal in vitreoretinal surgical techniques for management of RVO. However, no definitive studies have reproducibly shown the visual benefit of vitrectomy with either AV sheathotomy, radial optic neurotomy, creation of a chorioretinal anastomosis or retinal vein cannulation thus far. Furthermore, the side-effect profile for all of these techniques includes vision-threatening complications, including choroidovitreal neovascularization, which may persist despite aggressive treatment in some eyes.
 |
With the introduction of anti-VEGF treatment and corticosteroid options, these more invasive surgical options have become less common in the armamentarium of treatment options for eyes with retinal vein occlusion.
However, PPV, removal of the posterior hyaloid and possible ILM peeling may derive added benefit to existing treatments, particularly in cases of persistent macular edema that would otherwise require frequent intravitreal treatment. The half-life of intravitreal anti-VEGF medications in vitrectomized eyes is shorter than in nonvitrectomized eyes, so they clear faster and have decreased efficacy.25 Thus, longer- acting adjuvant treatment, such as more durable anti-VEGF agents or corticosteroids, may need to be considered after PPV in eyes with RVO.
Bottom line
The known anatomical improvement from PPV with or without ILM peeling should be balanced with the likelihood of associated visual improvement. We should consider this in the context of several other factors, such as retinal perfusion status, the length of time since the initial event and concurrent ophthalmic comorbidities that may limit visual acuity. RS
REFERENCES
1. Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol. 1988;106:1469-1471.
2. Opremcak EM, Bruce RA. Surgical decompression of branch retinal vein occlusion via arteriovenous crossing sheathotomy: a prospective review of 15 cases. Retina. 1999;19:1-5.
3. Cahill MT, Kaiser PK, Sears JE, Fekrat S. The effect of arteriovenous sheathotomy on cystoid macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2003;87:1329-1332.
4. Cahill MT, Fekrat S. Arteriovenous sheathotomy for branch retinal vein occlusion. Ophthalmol Clin North Am. 2002;15:417-423.
5. Shuler RK, Jr., Fekrat S. Does radial optic neurotomy alter retinal blood flow in eyes with a central retinal vein occlusion? Am J Ophthalmol. 2006;141:145-146.
6. Weizer JS, Stinnett SS, Fekrat S. Radial optic neurotomy as treatment for central retinal vein occlusion. Am J Ophthalmol. 2003;136:814-819.
7. Aggermann T, Brunner S, Krebs I, et al. A prospective, randomised, multicenter trial for surgical treatment of central retinal vein occlusion: results of the Radial Optic Neurotomy for Central Vein Occlusion (ROVO) study group. Graefes Arch Clin Exp Ophthalmol. 2013;251:1065-1072.
8. Fekrat S, de Juan E, Jr. Chorioretinal venous anastomosis for central retinal vein occlusion: transvitreal venipuncture. Ophthalmic Surg Lasers. 1999;30:52-55.
9. Bynoe LA, Hutchins RK, Lazarus HS, Friedberg MA. Retinal endovascular surgery for central retinal vein occlusion: initial experience of four surgeons. Retina. 2005;25:625-632.
10. de Smet MD, Meenink TC, Janssens T, et al. Robotic Assisted Cannulation of Occluded Retinal Veins. PLoS One. 2016;11:e0162037.
11. Willekens K, Gijbels A, Schoevaerdts L, et al. Robot-assisted retinal vein cannulation in an in vivo porcine retinal vein occlusion model. Acta Ophthalmol. 2017;95:270-275.
12. Gijbels A, Smits J, Schoevaerdts L, et al. In-Human robot-assisted retinal vein cannulation, a world first. Ann Biomed Eng. 2018;46:1676-1685.
13. Nishida A, Kojima H, Kameda T, Mandai M, Kurimoto Y. Five-year outcomes of pars plana vitrectomy for macular edema associated with branch retinal vein occlusion. Clin Ophthalmol. 2017;11:369-375.
14. Noma H, Funatsu H, Mimura T, Eguchi S, Shimada K. Visual acuity and foveal thickness after vitrectomy for macular edema associated with branch retinal vein occlusion: A case series. BMC Ophthalmol. 2010;10:11.
15. Noma H, Funatsu H, Mimura T, Shimada K. Visual acuity and foveal thickness after vitrectomy for macular edema. Ophthalmologica. 2010;224:367-373.
16. Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87-93.
17. Praidou A, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, Dimitrakos S. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89(248-254.
18. Matsunaga N, Chikaraishi Y, Izuta H, et al. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology. 2008;115:1916-1922.
19. Stefansson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31:284-289.
20. Hikichi T, Konno S, Trempe CL. Role of the vitreous in central retinal vein occlusion. Retina. 1995;15:29-33.
21. Wolf S, Schnurbusch U, Wiedemann P, Grosche J, Reichenbach A, Wolburg H. Peeling of the basal membrane in the human retina: ultrastructural effects. Ophthalmology. 2004;111:238-243.
22. Adelman RA, Parnes AJ, Bopp S, Saad Othman I, Ducournau D. Strategy for the management of macular edema in retinal vein occlusion: the European VitreoRetinal Society macular edema study. Biomed Res Int. 2015;2015:870987.
23. DeCroos FC, Shuler RK, Jr., Stinnett S, Fekrat S. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147:627-633 e621.
24. Radetzky S, Walter P, Fauser S, Koizumi K, Kirchhof B, Joussen AM. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273-278.
25. Edington M, Connolly J, Chong NV. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol. 2017;13:1217-1224.



