 |
Here, we summarize five of the most interesting presentations. They include a new medication for the slowing of geographic atrophy, the subretinal injection of a bioengineered retinal pigment epithelium monolayer for advanced GA, the utility and safety of flanged haptics in sutureless scleral fixation of intraocular lenses, the outcomes of patients with proliferative diabetic retinopathy after being lost to follow-up, and the use of a port delivery system (PDS) for neovascular age-related macular degeneration.
Complement C3 Inhibitor for GA
The treatment of GA in AMD has perplexed retina specialists for decades. Fortunately, there appears to be significant promise with APL-2 (Apellis Pharmaceuticals), a complement C3 inhibitor, in slowing the growth of GA. APL-2, or pegcetacoplan 15 mg, is a synthetic cyclic peptide conjugated to a polyethylene glycol polymer that binds specifically to C3 in the complement pathway. C3 resides far “upstream” in the complement pathway; thus, its inhibition by APL-2 impedes all three pathways of complement activation—classical, lectin and alternative.
In this prospective, randomized, sham-controlled, single-masked Phase II trial,1 patients with GA area measuring 2.5 to 17.5 mm2 and best-corrected visual acuity of >24 letters (20/320 Snellen equivalent) were randomized to four arms: APL-2 monthly (n=86); APL-2 every other month (EOM; n=79); sham monthly (n=41); and sham EOM (n=40). The patients were treated for 12 months, followed by six months of observation. The primary outcome was the difference in mean change from baseline in square-root GA area based on fundus autofluorescence at 12 months.
 |
A potentially concerning finding was a higher rate of conversion to neovascular AMD in the treatment groups: 20.9 percent in the APL-2 monthly group; 8.9 percent in the APL-2 EOM group; and 1.2 percent in the sham pooled group.
Previous attempts at targeting the complement pathway to slow the progression of GA in AMD have failed. However, this Phase II trial of APL-2 has shown impressive results, and the retina community hopes it becomes the first Food and Drug Administration-approved treatment for GA. The higher rates of conversion to nAMD in the treatment groups are concerning, but larger trials are needed to understand the possible association with choroidal neovascularization. Phase III clinical trials will begin this year.
Stem Cell RPE Monolayer
APL-2 may help to slow growth of GA, but it doesn’t result in any improvement to the area where the RPE has already been lost. Amir Kashani, MD, and colleagues aimed potentially to improve vision in patients with GA by implanting a monolayer of RPE under the retina.2
They created a clinical-grade retinal implant consisting of a polarized monolayer of human embryonic stem cell-derived RPE grown on a synthetic substrate that was designed to mimic Bruch’s membrane. They are currently engaged in a Phase I/IIa study to assess the safety and efficacy of a composite subretinal implant in patients with advanced GA.
The interim results analyzed four subjects that successfully received the implant in a surgical procedure. It appeared to be safe and well tolerated. In all four subjects, optical coherence tomography demonstrated integration of the RPE implant with host photoreceptors. None of the eyes demonstrated worsening of vision. Astonishingly, one subject’s vision in the implanted eye improved by 17 letters. Two eyes were reported to have improved fixation.
This implant has the potential to be the first treatment for dry AMD that may result in improved vision, a groundbreaking step for a disease that remains without a viable treatment. Additional studies with larger populations are needed to better evaluate its safety and efficacy, but the preliminary results are very encouraging.
 |
Sutureless intrascleral (SIS) fixation of intraocular lenses has become an increasingly popular surgical technique in eyes with inadequate capsular support. In this study, the authors demonstrated that the additional step of “haptic flanging” helps to minimize postoperative IOL dislocation in these cases. Haptic flanging involves using low-temperature cautery to melt the distal tips of both haptics of the three-piece IOL used for sutureless scleral fixation.
The authors initially performed 27-gauge SIS fixation of a three-piece IOL on five cadaveric human eyes. They compared the force required to dislocate a flanged and unflanged haptic. In these five eyes, flanged haptics required significantly more force to dislocate the IOL than unflanged haptics (14 ±4 vs. 3 ±1 g, p=0.03).
A retrospective review of 52 SIS cases with haptic flanging reported a mean visual acuity gain from 20/140 to 20/50 one month postoperatively (p<0.001). The most common postoperative complication was intraocular pressure rise (n=12, 23 percent).
The additional step of haptic flanging during SIS surgery creates a more stable scleral-fixated IOL compared with the traditional unflanged technique based on the cadaveric human eye study. This study provides valuable data to support haptic flanging to reduce the rate of IOL dislocation.
PRP vs. Anti-VEGF for LTFU
Most retina specialists are well versed in the results of the Diabetic Retinopathy Clinical Research Network Protocol S study, which found that the treatment of proliferative diabetic retinopathy with intravitreal ranibizumab (Lucentis, Roche/Genentech) was non-inferior to panretinal photocoagulation with respect to visual outcomes at two years. In this study, the authors examined a “real-world” scenario that is difficult to evaluate in a prospective clinical trial: loss to follow-up (LTFU) after treatment for PDR.4
This retrospective study included 76 eyes of 59 patients who were LTFU for more than six months after initial treatment of either IVT anti-VEGF (30 total eyes) or PRP (46 eyes) for PDR. Between the two treatment groups, the study gathered and compared visual-acuity and anatomic outcomes at the visit before LTFU, return visit, sixand 12-month visits after return, and the final visit.
In both the IVT and PRP eyes, mean logMAR VA significantly worsened when comparing the visit before LTFU to the return visit. When comparing VA at the final visit to the visit before LTFU, IVT eyes demonstrated significantly worse VA (0.92 [±0.94], 20/166, p=0.01), while PRP eyes showed no significant difference in VA (0.46 [±0.47] 20/58, p=0.38).
Furthermore, the incidence of tractional retinal detachments was significantly higher in the IVT group at the return visit (five vs. none, p=0.008) and the final visit (10 vs. one, p=0.005). The IVT group also had significantly greater incidence of iris neovascularization at the final visit (four vs. none, p=0.02). Eyes with PDR that received only IVT and were subsequently LTFU demonstrated worse visual and anatomical outcomes than those that received PRP.
These very intriguing findings encourage us not to take the outcomes of a clinical trial such as Protocol S completely at face value. Despite the advantages of IVT compared to PRP for PDR that Protocol S demonstrated (superior improvement in VA in eyes with concurrent diabetic macular edema, less visual field loss and lower rates of vitrectomy), we must note that these outcomes occurred only with regular follow-up and treatment in the context of a well-organized clinical trial.
Unfortunately, the compliance of diabetic patients in our clinics can be far from predictable, as many of them attempt to balance occupational and family obligations with the burden of numerous doctor visits. This study highlights that we must consider the possibility of LTFU when determining treatment—and that we should still consider PRP as a viable treatment option, particularly in patients who are unable to consistently return for appropriate follow-up.
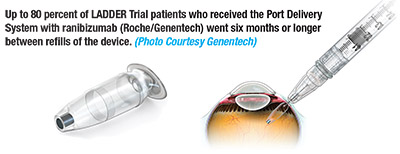
Ranibizumab PDS
The treatment burden associated with appropriate care of nAMD is a problem that must be addressed in the coming years, particularly with the rapid growth of the aging population in the United States. The Port Delivery System (PDS) with ranibizumab (Roche/Genentech) aims to address this challenge.
The small, refillable PDS implant is inserted at the pars plana in an outpatient procedure and is filled with a concentrated formulation of ranibizumab at the time of surgery. It can subsequently be refilled during a minor office procedure similar to a standard intravitreal injection. Phase II trial results for the PDS show great promise in significantly reducing treatment burden while also maintaining excellent outcomes.
In this prospective, multicenter, randomized, interventional, active treatment-controlled trial,5 179 patients were randomized to receive the PDS implant with a dose of either 10 mg/mL (n=58), 40 mg/mL (n=62), or 100 mg/mL (n=59) of ranibizumab. In the 100-mg/mL group, approximately 80 percent went six months or more without needing a refill of medication. In the 10-mg/mL and 40mg/mL groups, 63.5 percent and 71.3 percent, respectively, went six months or more. When compared to a control group of patients receiving the standard monthly injections of ranibizumab, the 100-mg/mL dose group demonstrated similar improvements in BCVA and reductions in measurements of central retina thickness on OCT.
The median time to requiring a refill for the 100 mg/mL group was 15 months.
Both patients and physicians would welcome reducing the need for monthly injections for nAMD. This trial demonstrates that the patients treated with the PDS can achieve visual and anatomic outcomes similar to our current gold-standard treatment of monthly injections. The retina community will be looking forward to the outcomes of the upcoming
Phase III trial of this device.
References
1.SteinleN,AbrahamP.APL-2,acomplementC3inhibitor,slows the growth of geographic atrophy secondary to AMD: 18-month results of a phase 2 trial (FILLY). Presented at American Society of Retina Specialists 2018; Vancouver, BC; July 21, 2018.
2. Kashani A, Rahhal AM, Humayan MS, et al. Subretinal implantation of a bioengineered embryonic stem cell-derived retinal pigment epithelium monolayer in dry age-related macular degeneration. Presented at American Society of Retina Specialists 2018; Vancouver, BC; July 21, 2018.
3. Stem M, Woodward M, Todorich B, Wolfe JD, Walsh MK, Way CA. 27-gauge sutureless intrascleral fixation of intraocular lenses with haptic flanging: clinical outcomes and a disinsertion force study. Presented at American Society of Retina Specialists 2018; Vancouver, BC; July 22, 2018.
4. Su D, Patel SN, Hsu J, Urh J, Obeid A. Outcomes in proliferative diabetic retinopathy patients lost to follow-up after panretinal photocoagulation versus antivascular endothelial growth factor. Presented at American Society of Retina Specialists 2018; Vancouver, BC; July 24, 2018.
5. Awh C, Regillo CD, Marcus DM, et al. LADDER trial of the port delivery system with ranibizumab: Initial study results. Presented at American Society of Retina Specialists 2018; Vancouver, BC; July 25, 2018.



