 |
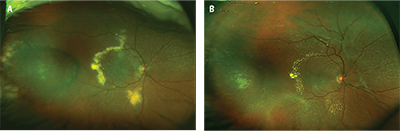
| Figure 1. Widefield fundus photography of the right eye shows new onset circinate exudates along the temporal macula with associated macula edema (A). Note the treated tumor in the temporal periphery. Treated macular edema (B) is visible with resolving exudates with new onset tractional membrane formation along the superior arcades. |
The patient’s ocular history was significant for choroidal melanoma of the right eye treated with proton-beam radiation 12 years previously. She had received routine annual ocular surveillance as well as systemic monitoring for liver metastases. Her most recent examination was nine months before the current visit and she was declared clinically stable with visual acuities of 20/20 bilaterally.
Fundus evaluation at that time revealed a stable, treated melanoma in the temporal periphery of the right eye, with extensive soft drusen in the macula of both eyes. An ultrasound of the right eye showed a minimally elevated lesion consistent with treated melanoma with a base diameter of 7 mm by 6 mm and height of 1.5 mm.
On the day of presentation, visual acuity had decreased to 20/400 in the right eye. Intraocular pressures, pupils and anterior segment examination were unremarkable. Fundus examination of the right eye demonstrated the previously noted drusen and treated tumor with a new area of circinate exudates along the superior and inferior arcades with associated macular edema (Figure 1A). A repeat ultrasound did not demonstrate a change in the size of the tumor. Optical coherence tomography confirmed the presence of intra- and subretinal fluid in the macula (Figure 2A, page 12).
Treatment Plan
Given this patient’s extensive macular edema, we initiated treatment with intravitreal bevacizumab (Avastin, Genentech). After three injections, she had recovery of visual acuity to 20/20 and OCT demonstrated complete resolution of the macular edema (Figure 2B, page 12).
However, in the subsequent months, the patient began to develop an area of fibrosis along the superior arcades with recurrent exudation (Figure 1B).
 |
| Figure 2. Optical coherence tomography of the right eye shows (A) intra-retinal and subretinal fluid with hard exudates extending to the optic nerve and (B) the resolution of fluid after a series of three monthly bevacizumab injections. |
Difficult Differential Diagnosis
The mainstay of globe-conserving therapy for uveal melanoma is radiation therapy, which can take the form of brachytherapy, proton-beam radiotherapy or stereotactic radiotherapy.1 Despite great success in salvaging the eye, the collateral damage from radiation treatment can cause significant morbidity within the eye and lead to permanent vision loss.
The pathophysiology of radiation retinopathy is predominantly secondary to the loss of vascular endothelial cells. The vascular compromise manifests clinically as microaneurysms, exudates, hemorrhages, cotton-wool spots, macular edema and diffuse retinal ischemia.2
Toxic tumor syndrome arises as a result of tumor necrosis and intratumoral radiation vasculopathy after treatment, leading to ischemia, vascular incompetence and severe exudation. This in turn can result in macular edema, retinal exudates, retinal detachment, iris neovascularization and neovascular glaucoma.
With small tumors, it is easier to completely obliterate the tissue and associated vasculature, resulting in an atrophic mass without metabolic activity. However, with bulky tumors, it is difficult to obtain such definitive treatment without excessive amounts of collateral damage to healthy tissue. Thus, bulky tumors may continue to release inflammatory cytokines and vascular growth factors for a prolonged period post-treatment.3
Based on the history and clinical findings, the differential diagnosis for this patient included radiation retinopathy vs. toxic tumor syndrome. Given the significant overlap between the two entities, differentiating between them can be difficult.
The time to onset of symptoms in cases of radiation retinopathy peaks at approximately two years,4 although late-onset radiation changes have been described even 15 years post-brachytherapy.5 Toxic tumor syndrome, on the other hand, does not have a well-characterized time course, but tends to have a more sudden, severe and often neovascular or proliferative presentation. We believe that our patient had components of both diseases.
| |
| Dr. Olmos de Koo is an assistant professor of ophthalmology at the University of Southern California Eye Institute and director of the vitreoretinal fellowship at the Keck School of Medicine of USC in Los Angeles. Dr. Pan is an ophthalmology resident at Keck School of Medicine; Dr. Aziz is a fellow at USC Eye Institute; and Dr. Berry is an assistant professor at USC Eye Institute who specializes in ocular onology. |
In the treatment of radiation retinopathy, laser photocoagulation, photodynamic therapy, intravitreal steroids and anti-vascular endothelial growth factor agents have all been employed. Only limited literature addresses laser photocoagulation and photodynamic therapy for treatment of radiation retinopathy. Intravitreal steroids and anti-VEGF agents, however, have been well-studied and shown to be effective.6 Despite adequate treatment, most patients suffer permanent visual compromise once radiation retinopathy is clinically evident.
The treatment of toxic tumor syndrome can be approached in two ways. The first approach is the same as that for radiation retinopathy. However, there appears to be a treatment bias toward periocular steroids given the inflammatory basis for the condition.7
The second approach is directed at reducing the treated tumor burden as the source of inflammation. In the case of smaller tumors, transpupillary thermotherapy can be used to definitely halt the neovascular drive, whereas for larger tumors endoresection or trans-scleral resection may be necessary to debulk the tissue.8 RS
REFERENCES
1. Shields JA, Shields CL, De Potter P, Singh AD. Diagnosis and treatment of uveal melanoma. Semin Oncol. 1996;23:763-767.
2. D’Amato B. Vasculopathy after treatment of choroidal melanoma. In: Joussen AM, Gardner TW, Kirchhof B, Ryan SJ, eds. Retinal Vascular Disease. Berlin and Heidelberg, Germany: Springer Berlin Heidelberg; 2007:582-591.
3. Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye. 2013;27:163-171.
4. Durkin SR, Roos D, Higgs B, Casson RJ, Selva D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. 2007;85:240-250.
5. Stallard HB. Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol. 1966;50:147-155.
6. Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21:233-238.
7. Parrozzani R, Pilotto E, Dario A, Miglionico G, Midena E. Intravitreal triamcinolone versus intravitreal bevacizumab in the treatment of exudative retinal detachment secondary to posterior uveal melanoma. Am J Ophthalmol. 2013;155:127-133.e2.
8. Damato BE. Local resection of uveal melanoma. Dev Ophthalmol. 2012;49:66-80.



