 |
 |
 |
The advent of anti-VEGF agents has been a remarkable success for the treatment of neovascular age-related macular degeneration, but despite significant research and investigation, no approved treatment exists for the much more common dry form of AMD that affects approximately 85 percent of patients1 and manifests as geographic atrophy in the late stage.2
GA can enlarge and may coalesce, resulting in irreversible paracentral or central scotoma, depending on the size and location of atrophy. Even parafoveal GA can cause substantial vision impairment with dark adaptation, reading and driving, and can affect quality of life.3 Here, we review past early phase GA trials that failed to show efficacy and current clinical trials that have shown promising results as potential treatments of GA.
Neuroprotective agents
Brimonidine is a selective alpha2 adrenergic receptor agonist that has long been used for the treatment of glaucoma. Animal studies have demonstrated that brimonidine is also neuroprotective for photoreceptors, bipolar cells and retinal ganglion cells against retinal phototoxicty and retinal ischemia through the release of brain-derived neurotrophic factor from retinal ganglion cells and by upregulation of the cell survival pathway. Thus, brimonidine has been investigated as a potential therapy for GA.4
The multicenter, randomized, double-masked, controlled Phase IIb BEACON study of 310 patients evaluated a second-generation Brimo DDS (Allergan) in a tartrate form, administered every three months compared with sham procedure in patients with GA (DDS stands for drug-delivery system).5 The implant showed a 7- percent reduction in GA area growth from baseline at month 24 and a statistically significant 11-percent reduction at month 30. Two definitive Phase III randomized, prospective multicenter studies of Brimo DDS at 200 µg and 400 µg doses are planned.5
Another investigative treatment, ciliary neurotrophic factor (CNTF), is a member of the interleukin-6 family of cytokines that is also a neurotrophic factor shown to slow the photoreceptor loss in animal models of retinal degeneration.6 In a pilot Phase II double-masked, sham-controlled dose-ranging trial, 51 patients with GA were randomized to CNTF delivered via intraocular encapsulated implant or sham surgery. The primary endpoint was change in best-corrected visual acuity at 12 months. While the CNTF group showed a statistically significant macular volume compared to controls, the treatment group showed no improvement in visual acuity or difference in GA area.7
 |
Mitochondrial enhancer
Elamipretide (Stealth Biotherapeutics) is a tetrapeptide believed to target cardiolipin in mitochondria, reduce reactive oxygen species production and increase adenosine triphosphate production in cells.
ReCLAIM was a Phase I open-label, single-site study that evaluated subcutaneous elamipretide for 24 weeks in patients with dry AMD with non-central GA or high-risk drusen. The initial study enrolled 40 patients and showed a significant improvement in low-luminance visual acuity and best-corrected visual acuity in the treatment arm. A randomized, double-masked, sham-controlled Phase IIb trial is under way and estimated to be completed by the end of 2020.8
Subthreshold nanosecond laser
Subthreshold nanosecond laser (SNL) initially showed promising results in vitro and in preclinical studies, as a single application of SNL resulted in reduction of drusen load without damage to overlying photoreceptors.9 The Laser Intervention in Early Stages of Age-Related Macular Degeneration (LEAD) study was a 36-month, randomized, sham-controlled trial of 292 patients with bilateral large drusen without any sign of atrophy on optical coherence tomography. The primary outcome was time to development of late AMD defined by multimodal imaging.
The study found that SNL treatment wasn't effective in slowing the progression from intermediate to late AMD. A post hoc analysis showed patients with reticular pseudodrusen (RPD) are at particularly increased risk of progression with SNL treatment. Further studies are warranted to elucidate the benefit of SNL in patients without RPD.10
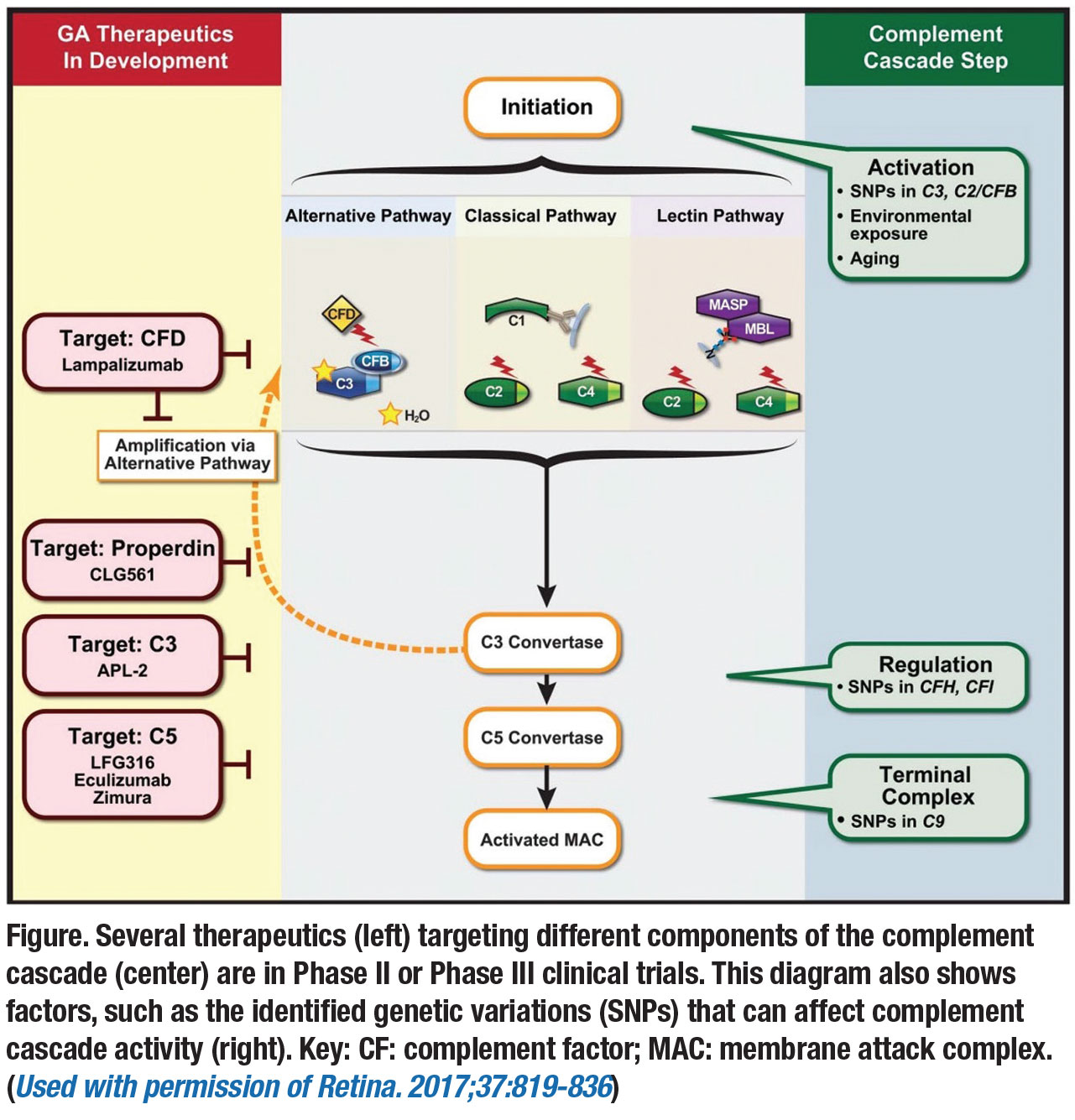
Complement inhibitors
This class of drugs includes the following investigational therapies:
• Lampalizumab (Roche/Genentech) is an antigen-binding fragment (Fab) of a humanized monoclonal antibody directed against complement factor D (CFD). It selectively inhibits the alternative complement pathway that has been implicated in the pathogenesis of GA. The initial MAHALO Phase II study showed promising results, as the treatment arm receiving monthly and every-other-month intravitreal lampalizumab had a 20-percent reduction in GA area progression compared to sham control.11
However, two Phase III, double-masked, randomized, sham-controlled clinical trials—Chroma and Spectri—failed to show reduction of GA growth compared to sham after 48 weeks and both studies were terminated.12 The trials randomized patients 2:1:2:1 to intravitreal lampalizumab every four weeks, sham procedure every four weeks, intravitreal lampalizumab every six weeks, and sham procedure every six weeks.
• Eculizumab (Soliris, Alexion Pharmaceuticals) is an inhibitor of complement component C5 but administered via intravenous infusion. In the Phase II COMPLETE study, 30 patients with GA were randomized to intravenous eculizumab or placebo over six months. The systemic medication was well-tolerated but didn't significantly decrease the growth rate of GA.13
• Avacincaptad pegol (Zimura, Iveric bio) is a polyethylene glycol, single-strand nucleic acid aptamer that targets and inhibits complement factor C5. Recruitment of a double-masked, randomized, sham-controlled Phase IIb clinical trial was completed with 286 patients with dry AMD with GA. Patients that received 2 mg or 4 mg of the drug experienced a mean reduction in GA growth of approximately 27 percent at 12 months compared to the sham group.14 Patients will continue to be treated with the study drug and followed through month 18 for additional data collection.
Multiple complement inhibitors
Pegcetacoplan is a pegylated peptide that binds specifically to C3 and blocks all three complement pathways (classical, lectin and alternative). APL-2 (Apellis) is a modified version of the peptide designed to have a longer half-life. In the prospective, multicenter, randomized, sham-controlled Phase II FILLY study, 256 patients with GA were randomized to APL-2 or sham intravitreal injections for 12 months and followed for an additional six months. The primary efficacy endpoint was mean change in square root GA lesion from baseline to month 12.
 |
The FILLY study met its primary endpoint, and patients receiving APL-2 monthly or every other month experienced a 29- and 20-percent reduction, respectively, in GA growth rate compared to the sham treatment group. A post-hoc analysis of 176 subjects that completed the 12-month visit with no missing data revealed that the FILLY trial population was a typical GA population, and that the benefit of APL-2 compared to sham remained statistically significant when the population was controlled for all key risk factors of GA progression.
However, pegcetacoplan eyes also had an increased incidence of exudative AMD; 21 and 9 percent in monthly and every-other-month groups compared to 1.2 percent in sham-treated eyes.15 Despite failures of previous complement inhibitors, there is cautious optimism for APL-2. Two Phase III trials (OAKS and DERBY) of 600 patients each, are under way; they're expected to be completed in 2022.
Bottom line
Improved understanding of GA pathophysiology will be necessary to help broaden the realm of potential therapeutic targets for the treatment of GA. In addition, more sophisticated methods for patient classification, through the use of machine or deep learning, and earlier identification of GA lesions by spectral-domain OCT will be of benefit, allowing detection of the subjects most likely to respond to promising GA therapies in the future. These breakthroughs could help structure successful clinical trials in GA. RS
REFERENCES
1. Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564-572.
2. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079-1091.
3. Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677-1691.
4. Saylor M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: An evidence-based review. Arch Ophthalmol. 2009;127:402-406.
5. Freeman WR. Brimonidine drug delivery system for geographic atrophy. Retinal Physician. 2019;16 (November/December): 42-51.
6. Fuhrmann S, Grabosch K, Kirsch M, Hofmann H-D. Distribution of CNTF receptor alpha protein in the central nervous system of the chick embryo. J Comp Neurol. 2003;461:111-122.
7. Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012;53:7484-7491.
8. Stealth BioTherapeutics announces positive results for elamipretide in ophthalmic conditions [press release]. BioSpace. April 30, 2019. https://www.biospace.com/article/stealth-biotherapeutics-announces-positive-results-for-elamipretide-in-ophthalmic-conditions/. Accessed November 22, 2019.
9. Jobling AI, Guymer RH, Vessey KA, et al. Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage. FASEB J. 2015;29:696-710.
10. Guymer RH, Wu Z, Hodgson LAB, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: The LEAD randomized controlled clinical trial. Ophthalmology. 2019;126:829-838.
11. Yaspan BL, Williams DF, Holz FG, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9:pil:eaaf1443. doi:10.1126/scitranslmed.aaf1443
12. Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri Phase III randomized clinical trials. JAMA Ophthalmol. 2018;136:666-677.
13. Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693-701.
14. Zimura Phase IIb update. Iveric bio [press release]. October 28, 2019. https://www.businesswire.com/news/home/20191028005259/en. Accessed 27 January 2020
15. Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized Phase II trial. Ophthalmology. 2019 July 16. doi:10.1016/j.ophtha.2019.07.011 [Epub ahead of print]



