 |
Advances in imaging technology and newly developed mathematical models have the potential to greatly advance our understanding of this most significant tissue and the role that it plays in the onset and progression of AMD. This is of critical importance for the design and implementation of better therapeutic strategies and monitoring tools for this disease.
Structure of the Choroid
The choroid is the sole supplier of oxygen and nutrients to the outer one-third of the retina, and it is the primary clearance route for retinal metabolism byproducts. Given the high metabolic rate of the photoreceptors—one of the highest of any cell of the human body—it is not
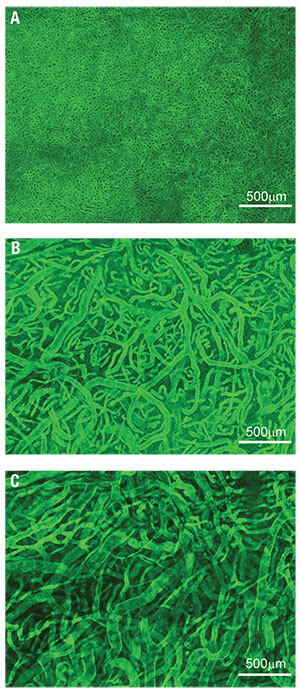 |
| Figure 1. The innermost vascular layer of the choroidal vasculature, here visualized in the submacular area, is the choriocapillaris (A), a densely organized capillary bed. Deeper inside the choroid, Sattler’s layer (B) is composed of arterioles and venules supplying the choriocapillaris. The outermost choroidal vascular layer, Haller’s layer (C), is made up of large arteries and veins. These images were obtained by immunostaining a portion of tissue dissected from a human eye and imaging them using confocal microscopy. |
Typically, four vortex veins drain each quadrant of the choroid and ciliary body and empty into the ophthalmic veins. The choroidal vasculature forms a segmented vascular tree, whereby branches of the choroidal arteries supply ever-smaller sectors of tissue and ultimately join the choroidal vascular bed, the choriocapillaris. Similarly, venules drain distinct sectors of the choriocapillaris and merge into larger veins.
This branching pattern gives a seemingly layered organization to the choroid in the posterior pole. The largest choroidal arteries and veins form the outermost vascular layer (Haller’s layer) and intermediate arterioles and venules make up the layer internal to Haller’s. The choriocapillaris and Bruch’s membrane, respectively, form the innermost layers of the tissue (Figure 1).1,2
The exchange between the retina and the choroid occurs mostly at the choriocapillaris, which, unlike most capillary beds, has evolved a planar vascular geometry.3 It forms a continuous and extensively anastomosing meshwork of capillaries supported by a rigid framework of intercapillary connective tissue that extends from the optic nerve margins to the ora serrata.
Capillaries are characteristically large in diameter; avascular septae or pillars separate them. Perhaps one of the most distinguishing features of choriocapillaris vessels is their fenestration; pores line their inner endothelium. The choriocapillaris attaches internally to Bruch’s membrane, which forms a complex five-layered structure between the choroid and the retinal pigment epithelium.1
Many aspects of the choroidal vasculature change with location in the eye. The vascular density of the choriocapillaris, which is the local volume fraction of tissue occupied by capillaries as opposed to septae, and the number of arterioles and veins inserting into the choriocapillaris are maximal at the macula. Bruch’s membrane is thicker in the submacular choroid as compared to the periphery of the eye.4,5 Enhanced-depth optical coherence tomography has shown that the choroid is at its thinnest at the fovea.6 Diurnal variations in choroidal thickness by up to 70 µm have been reported;7 however, the mechanisms involved in this regulation are yet to be identified.
Dramatic changes in the structure of the choroid have been reported with normal aging. Between the ages of 6 and 100, the choriocapillaris vascular density and choroidal thickness decrease on average by 45 percent and 57 percent, respectively. Bruch’s membrane undergoes the largest changes, with an increase of 135 percent in thickness on average.8
Unique Functional Features
The choroid has evolved into a structure capable of supporting a variety of transport processes with great efficiency (Figure 2). The delivery of oxygen to the photoreceptors relies on a steep gradient of concentration that drives oxygen from the choriocapillaris across the outer retina. This gradient requires a high blood flow in the choroid.9,10 It is one of the largest in the human body with a per-unit mass flow three to four times greater than that in the kidney and about 10 times that of the brain.
Because choriocapillaris vessels are fenestrated, they are highly permeable to many low-molecular weight substances such as glucose.11 This helps maintain a high concentration at the interface between the choroid and the retina and facilitates transport to and across the RPE. The choriocapillaris also participates in maintaining an adequate oncotic pressure in the extravascular choroidal tissue, which is key to ensuring proper fluid movement and drainage. In addition to sustaining the outer retina, some have suggested that the high choroidal blood flow plays a key role in the thermoregulation of the back of the eye,12 thus protecting the retina from damage in extreme temperatures.
A unique and striking functional feature of the choroid is that the blood flow in the choriocapillaris is decomposed into a tessellation of functional lobules, which fill independently from each other. This segmentation, which is observable during dye angiography of the choroid (Figure 3), has recently been shown to be a natural consequence of the continuity of the choriocapillaris and the way in which arterioles and venules insert into the plane of the capillaries.3
Choroidal Changes in AMD
AMD is a complex disease with genetic22 and aging components. Evidence suggests that it involves a breakdown of mass exchange processes within and between the choroid and retina. We do not understand how this breakdown initiates or evolves as AMD progresses; however, exploring changes in the retina and choroid in both health and disease is key to identifying the factors involved.
Many structural
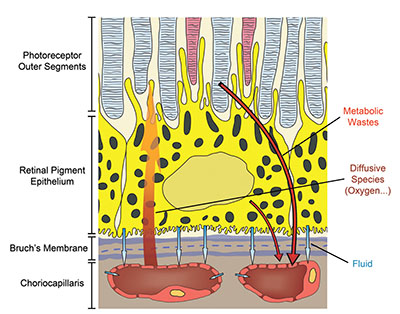 |
| Figure 2. This schematic illustrates the variety of transport processes crucial to vision that the choroid sustains. These processes include the supply of passive compounds, such as oxygen, to the photoreceptors, the maintenance of fluid movement in the back of the eye and the clearance of various retinal metabolic wastes. |
In eyes with GA, complete atrophy of the RPE is associated with a significant loss of choriocapillaris density. Remaining capillaries are largely non-fenestrated and are extremely constricted, which suggests that choriocapillaris function is dramatically impaired. In eyes with choroidal neovascularisation, a reduced choriocapillaris density is observed near the CNVs in the absence of RPE atrophy.15 Of potential significance, given the genetic risk associated with dysregulation of the alternative complement pathways, is the localization of complement components in the inner choroid, including in the region of the choriocapillaris.16
Few functional changes have been reported in the choroid in AMD, and they are often difficult to reconcile with structural changes. Early AMD is associated with a prolonged choroidal filling phase during angiography, which may result from a reduced choroidal perfusion or changes in the diffusional properties of Bruch’s membrane that the presence of diffuse deposits may cause.17 The distribution of reticular pseudodrusen has been found to relate to watershed zones of the choroidal vasculature,18 which would indicate a possible link between their formation and choroidal perfusion.
Computational Models: Insight Into Structure and Function
An important challenge in AMD research is to understand how structural and functional changes affect the physiology of the choroid and its ability to sustain the outer retina. Numerous aspects to consider in both the choroid and retina and the difficulty of accessing the back of the eye make conventional methods insufficient or difficult to implement.
Computational methods are particularly well suited to investigate complex systems and have the potential to radically improve our understanding of the dynamics of the choroid in health and disease. Basic physical principles and eighteenth century mathematics have helped show that blood flow in the choriocapillaris is significantly heterogeneous and decomposes into non-communicating functional vascular segments that correspond to functional lobules. The boundaries between functional lobules consist of regions, where the transport of passive compounds is dominantly diffusive and, therefore, comparatively slower (Figure 3).
Regions of comparatively lower perfusion are omnipresent over the choriocapillaris and their number is maximal in the submacular area, which could indicate a higher susceptibility to changes in this region. Evidence has shown that the shape of functional lobules is a key determinant in the exchange of diffusive species between the choroid and the outer retina and that it decreases approximately linearly with the choriocapillaris vascular fraction.3
Variations in the vascular density of the choriocapillaris and the relative distribution of arterioles and venules inserting into the choriocapillaris may allow for a local tuning of the perfusion pressure and mass extraction.3,19
Only a few mathematical models have explored the effect of pathologies associated with AMD theoretically. A mathematical investigation of CNV dynamics has suggested that a reduction in choriocapillaris blood flow may be sufficient to reduce CNVs and associated retinal edema.20 Variations in the distance between the photoreceptors and the choriocapillaris have been shown to have a dramatic effect on oxygen tension at the photoreceptor inner segments.21
A mathematical model of the RPE/Bruch’s membrane/choriocapillaris complex has revealed that, rather than being driven by growth factors or holes in Bruch’s membrane, the initiation and progression of CNVs are dominantly driven by defects in RPE adhesion to Bruch’s membrane.22
Challenges and Future Directions
A major challenge of AMD research is to identify the chain of biological events that lead to the onset and progression of the disease. This is essential not only for the early diagnosis and monitoring of AMD, but also to determine when, where and how to intervene. Genetic variations in several RPE genes are strongly associated with AMD,13 which indicates that the RPE plays a key role in the onset of the disease. Based on histological observations, some authors have suggested that choroidal failure is in fact the main trigger of AMD.23
In animal models, failure of the choroid leads to retinal degeneration. However, evidence also suggest that retinal degeneration results in choriocapillaris loss. Many studies have shown that biological markers of AMD are present in the retina and the choroid,16,24 which suggest functional impairments in both tissues. This evidence indicates that the retina and the choroid should ideally be considered together to identify the key events in the onset and progression of AMD. These investigations also demonstrate that a better understanding of the complexity of the interactions between the two tissues is crucial.
While we have focused on AMD, it is worth noting that choroidal changes have a role in other conditions, such as diabetes and hypertension. It will be important to understand the similarities and differences between these conditions and, in the case of diabetic eye disease, to what extent choroidal
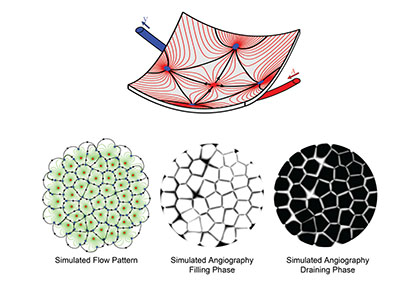 |
| Figure 3. Computational models may be used to simulate the blood flow and dye angiography in the choriocapillaris. In this computationally simulated blood flow pattern, blood travels along the lines connecting arterioles (red dots) to venules (blue dots). Because of the geometry of the choriocapillaris, the blood flow is segmented into a tessellation of functional lobules, which are observable during the filling and draining phases of angiograms. The boundaries between lobules correspond to regions where the travel time of the dye is comparatively longer. |
While still a developing technology, OCT angiography has the potential to radically improve our understanding of retinal and choroidal structures and function, and how they change with age and in AMD.25 When combined with other conventional imaging techniques, OCT-A may provide a window into the relationship between choroidal and retinal changes in AMD. Advances in OCT-A or possibly other imaging technology are likely to make it possible to establish absolute rates of flow in the choriocapillaris and larger choroidal vessels which will be even more powerful.
Computational methods and systems approaches offer powerful platforms to develop an integrated understanding of the retina and choroid and allow researchers to investigate the interactions between the two tissues. At a time when mining the vast amount of everyday, clinical data has become a challenge, computational models offer an ideal framework in which various clinical observations may be brought together to simulate retinal and choroidal function.
The assessment of the choriocapillaris function may, for instance, be improved by combining angiography of the choroid with novel mathematical frameworks.3 Cross-disciplinary collaborations may be challenging, but they are key to tackling this complex and multifactorial disease.
Take-home Point
Advances in imaging are shedding more light on the critical role choroidal failure plays in the pathogenesis of age-related macular degeneration. This article describes what has been learned about choroidal anatomy in recent years, its function in delivering oxygen to photoreceptors, and what changes the choroid undergoes in the progression of AMD. The authors also discuss how computational models are advancing the understanding of disease progression and how they may influence AMD management in the future. RS
REFERENCES
1. J. Hogan M, A. Alvarado J, Esperson Weddell J. Histology of the Human Eye: An Atlas and Textbook. W. B. Sauders Company; 1971:320.
2. Hayreh SS. Physiological anatomy of the choroidal vascular bed. Int Ophthalmol. 1983;6:85-93.
3. Zouache MA, Eames I, Klettner CA, Luthert PJ. Form, shape and function: segmented blood flow in the choriocapillaris. Sci Rep. 2016;6:35754.
4. Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724-2735.
5. Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44 Suppl 1:S10-32.
6. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811-815.
7. Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53:2300-2307.
8. Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857-2864.
9. Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol. 1992;99:177-197.
10. Linsenmeier RA, Goldstick TK, Blum RS, Enroth-Cugell C. Estimation of retinal oxygen transients from measurements made in the vitreous humor. Exp Eye Res. 1981;32:369-379.
11. Bill A, Törnquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100:332-336.
12. Bill A. Intraocular pressure and blood flow through the uvea. Arch Ophthalmol. 1962;67:336-348.
13. Black JRM, Clark SJ. Age-related macular degeneration: genome-wide association studies to translation. Genet Med. 2016;18:283-289.
14. Hillenkamp J, Hussain AA, Jackson TL, Cunningham JR, Marshall J. The influence of path length and matrix components on aging characteristics of transport between the choroid and the outer retina. Invest Ophthalmol Vis Sci. 2004;45:1493-1498.
15. Bhutto I, A. Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33;295-317.
16. Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006;103:17456-17461.
17. Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353-1358.
18. Alten F, Clemens CR, Heiduschka P, Eter N. Localized reticular pseudodrusen and their topographic relation to choroidal watershed zones and changes in choroidal volumes. Invest Ophthalmol Vis Sci. 2013;54:3250-3257.
19. Zouache MA, Eames I, Luthert PJ. Blood flow in the choriocapillaris. J Fluid Mech. 2015;774:37-66.
20. Flower RW, Kerczek C von, Zhu L, Ernest A, Eggleton C, Topoleski LD. Theoretical investigation of the role of choriocapillaris blood flow in treatment of subfoveal choroidal neovascularization associated with age-related macular degeneration. Am J Ophthalmol. 2001;132:85-93.
21. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41:3117-3123.
22. Shirinifard A, Glazier JA, Swat M, et al. Adhesion failures determine the pattern of choroidal neovascularization in the eye: a computer simulation study. PLoS Comput Biol. 2012;8:e1002440.
23. Mullins RF, Khanna A, Schoo DP, et al. Is age-related macular degeneration a microvascular disease? Adv Exp Med Biol. 2014;801:283-289.
24. Hageman G. An Integrated hypothesis that considers drusen as biomarkers of iummune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705-732.
25. Kashani AH, Chen C-L, Gahm JK, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017.



