Anti-VEGF medications have been revolutionary for the treatment of neovascular, or exudative, age-related macular degeneration, but unmet needs continue. First, intravitreal injections require frequent and indefinite evaluations, with a particularly high burden during the first two years of treatment.1 Second, current treatments have a short duration of effect. Third, despite robust response and visual gains in many patients, up to 30 percent may continue to lose vision from baseline due to inadequate choroidal neovascularization response, progression of atrophy2 or subretinal fibrosis and scar formation. Finally, with long-term follow-up, more than half of patients may have vision worse than 20/40, which may limit their daily activities despite anti-VEGF treatment.3
 |
To address these unmet needs, significant research into improving outcomes in neovascular AMD continues. Current treatments under investigation include new, longer-acting anti-VEGF agents, sustained-delivery systems, gene therapy and molecules targeting novel angiogenesis checkpoints. This article highlights a few of the most promising approaches currently under investigation.
Longer-Acting Anti-VEGF Drugs
Newer anti-VEGF agents are under investigation that may require less frequent dosing, including brolucizumab (RTH258, Novartis)4 and abicipar pegol (Allergan and Molecular Partners).5
• Brolucizumab. This humanized, single-chain antibody of 26 kDa, which is a smaller molecule than ranibizumab (48k Da) and aflibercept (115 kDa), has a high affinity for VEGF (Table).4 The molecular properties, including its small size, lead to a fast systemic clearance and ability to deliver a high molar content in a 0.05-cc intravitreal injection volume. The multicenter Phase III HAWK and HARRIER trials compared intravitreal aflibercept (Eylea, Regeneron) 2 mg to intravitreal brolucizumab 3 mg and 6 mg in more than 1,800 patients.
After three monthly loading doses, the 6-mg brolucizumab group met the primary endpoint of noninferiority to aflibercept every eight weeks as measured by mean change in best-corrected visual acuity from baseline to week 48. In the HARRIER and HAWK trials, 52 percent and 57 percent of patients, respectively, were maintained at a 12-week dosing interval after initial monthly loading doses through week 48. Intravitreal brolucizumab was well tolerated, and the rate of ocular and non-ocular adverse events was comparable to aflibercept.
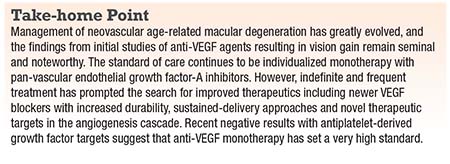
• Abicipar pegol. This is a DARPin, or designed ankyrin repeat protein (Figure 1, page 16)—a novel class of synthetic, small-binding proteins with high affinity and specificity that blocks all isoforms of VEGF-A, along with very long half-life of up to two weeks.5 In the Phase II REACH studies, intravitreal abicipar 2 mg monthly for three loading doses outperformed monthly intravitreal ranibizumab 0.5 mg (Lucentis, Roche/Genentech) at a 20-week endpoint, with the abicipar group gaining 9 letters and the ranibizumab group gaining 4.7 letters.
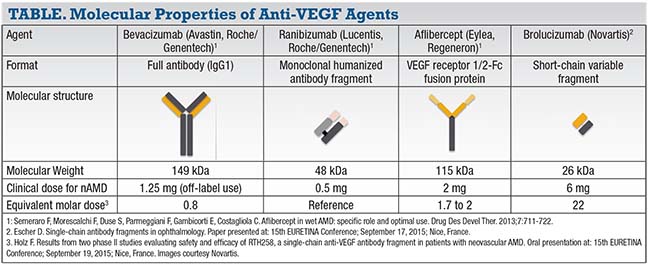 |
Of note, the gain of vision with ranibizumab in previous trials with monthly dosing, including MARINA, ANCHOR and CATT, showed a vision gain of 6 to 10 Early Treatment Diabetic Retinopathy Study (ETDRS) letters. In the Phase II trial of abicipar, 10 percent of patients experienced episodes of ocular inflammation, which has also been observed with aflibercept in clinical practice and may be reduced with improved formulation of abicipar and purification during the manufacturing process. Two Phase III trials, CEDAR and SEQUIOA, comparing abicipar 2 mg dosed at eight and 12 weeks and ranibizumab at four weeks are now fully enrolled with results expected later in the year.
Sustained-delivery Treatments
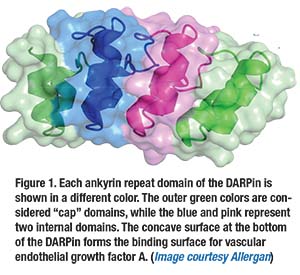
Another strategy to address the treatment burden of frequent anti-VEGF injections is sustained delivery. Encapsulated cell technology, such as the NT-503 vitreous implant (Neurotech), with encapsulated retinal pigment epithelium cells that produce a vascular endothelial growth factor receptor for up to two years, have been investigated. Other, more recent sustained-delivery implants include the ranibizumab Port Delivery System (RPDS, Roche/Genentech) and the Posterior MicroPump Drug Delivery System (Replenish Inc.).6 Another sustained-release technology is GB-102 sunitinib maleate (GrayBug Vision), a non-inflammatory biodegradable polymer that is injected via a 27- or 29-gauge needle and inhibits VEGF-A and platelet-derived growth factor (PDGF).
 |
RPDS is a non-biodegradable intraocular reservoir that is inserted via a 3.2-mm pars plana incision in the operating room. It allows delivery of concentrated ranibizumab over four to six months (Figure 2).7 The 50-µl reservoir can be refilled in the office with a proprietary syringe to access the device. Phase I results have shown a vision gain comparable to monthly
intravitreal ranibizumab; however, some cases of postoperative vitreous hemorrhage have been reported.
The surgical procedure has been modified to deliver laser treatment to the bed of the scleral incision during insertion to decrease the
incidence of post-procedure vitreous hemorrhage. The Phase II
LADDER trial is currently fully enrolled with approximately 220 patients at 45 sites randomized to three doses of ranibizumab in the RPDS device (10 mg/mL, 40 mg/mL, 100 mg/mL) vs. monthly ranibizumab. The primary outcome is timing to first refill of the RPDS.
Gene Therapy
Gene therapy for RPE65 mutations in Leber congenital amaurosis (LCA) recently received Food and Drug Administration approval,8 and interest in this type of therapy for treatment of nAMD continues to grow.9 Gene therapy uses a viral vector as conduit for transferring genes that instruct the host cells to produce therapeutic proteins that bind VEGF. It offers the promise of long-term, durable expression of anti-VEGF proteins after a single administration.
• AVA-101 (Adverum Biotechologies Inc.). This adenoviral-associated virus (AAV) vector has been delivered via pars plana vitrectomy and subretinal injection. It produces sFLT-1, a tyrosine kinase inhibitor that binds VEGF.10 Phase II data of 32 patients (21 treatment, 11 control), showed a wide distribution of outcomes, including a subset of patients who showed signs of encouraging activity.11 The median number of rescue injections using the protocol-specified re-treatment regimen was two in AVA-101-treated subjects compared with four in the control group.
• RGX-314 (RegenxBio Inc.). An investigational AAV8 gene therapy being developed for patients with nAMD, RGX-314 contains a transgene that leads to the production of anti-VEGF fab protein that exhibits anti-VEGF activity similar to ranibizumab. A Phase I multiple cohort, dose-escalation study to evaluate the safety and tolerability of gene therapy with RGX-314 in patients with nAMD is underway. The primary endpoint is safety of RGX-314 through week 26 with incidence of ocular and non-ocular adverse events (AEs) and serious AEs.
 |
The study is currently enrolling with a goal of 18 participants who will receive three dose cohorts of RGX-314 (3 x 109 GC/eye, 1 x 1010 GC/eye and 6 x 1010 GC/eye). Subjects with nAMD receive intravitreal ranibizumab 0.5 mg on day one and will be evaluated by spectral-domain optical coherence tomography one week later to confirm anatomic response to initial anti-VEGF activity, and then at week two will receive a single dose of RGX-314 via pars plana vitrectomy with a subretinal injection.
Novel Angiogenesis Targets
Another therapeutic approach is targeting other parts of the angiogenesis cascade beyond VEGF, which are numerous and include targets such as growth factor, interleukin, angiopoeitin and matrix metalloproteinases.
Combination Therapies
The much-anticipated results of combination therapy with inhibitors of PDGF, including Fovista (Ophthotech Corporation) and rinucumab (Regeneron) in combination with anti-VEGF therapy, have failed to show superiority over anti-VEGF alone.12
• Squalamine. Squalamine lactate 0.2% (Ohr Pharmaceuticals) is a small-molecule agent that inhibits not only anti-VEGF but also PDGF and fibroblast growth factor though the modulation of the protein calmodulin.13 While intravenous squalamine has been poorly tolerated, the exploratory Phase II IMPACT trial suggested topical squalamine b.i.d. provided a 65 percent added benefit to ranibizumab p.r.n. However, given the disappointing results of intravitreal PDGF inhibition, the sponsor does not plan to continue with a Phase III trial at this time.
 |
Current combination studies are investigating compounds that inhibit anti-VEGF and downregulate angiopoietin 2. They include the bispecific antibody RG7116 (Roche/Genentech) and nesvacumab (Regeneron) co-administered with aflibercept.
• Targeted immunotherapy. In this novel approach, vascular endothelial cells and macrophages express tissue factor (TF) in choroidal neovascularization but not normal micro-vasculature.14 ICON-1 (Iconic Therapeutics) is a recombinant human fusion protein (composed of factor VII and antibody) that binds TF. Binding of ICON-1 then triggers an endogenous cytotoxic effect via natural killer cells to regress and destroy choroidal neovascularization. Phase II EMERGE results have suggested that intravitreal ICON-1 in combination with ranibizumab enhances the durability but not the efficacy of ranibizumab monotherapy with less re-treatment and longer time to re-treatment. 15
What About Dry AMD?
It is important to note that significant advances in the treatment of dry or nonexudative AMD also represent an unmet clinical need. Although there is a clear and highly significant genetic association with AMD and complement factor H,16 attempts to turn this into a successful therapeutic target have not materialized.17 Failed efforts have included targeting factor D,18 but clinical trials are currently underway investigating C5 inhibition (Zimura, Ophthotech), C3 inhibition (APL-2, Apellis) and cell therapies, including stem-cell suspensions (Astellas Pharma) and polarized parylene monolayers.19 RS
REFERENCES
1. Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS One. 2015;10:e0133968.
2. Munk MR, Ceklic L, Ebneter A, Huf W, Wolf S, Zinkernagel MS. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2016;94:e757-e764.
3. Peden MC, Suner IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803-808.
4. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: A randomized trial. Ophthalmology. 2017;124:1296-1304.
5. Smithwick E, Stewart MW. Designed ankyrin repeat proteins: A look at their evolving use in medicine with a focus on the treatment of chorioretinal vascular disorders. Antiinflamm Antiallergy Agents Med Chem. 2017;16:33-45.
6. Humayun M, Santos A, Altamirano JC, et al. Implantable MicroPump for drug delivery in patients with diabetic macular edema. Transl Vis Sci Technol. 2014;3:5.
7. Smith AG, Kaiser PK. Emerging treatments for wet age-related macular degeneration. Expert Opin Emerg Drugs. 2014;19:157-164.
8. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849-860.
9. Moore NA, Bracha P, Hussain RM, Morral N, Ciulla TA. Gene therapy for age-related macular degeneration. Expert Opin Biol Ther. 2017;17:1235-1244.
10. Constable IJ, Lai CM, Magno AL, et al. Gene therapy in neovascular age-related macular degeneration: Three-year follow-up of a Phase 1 randomized dose escalation trial. Am J Ophthalmol. 2017;177:150-158.
11. Constable IJ, Pierce CM, Lai CM, et al. Phase 2a randomized clinical trial: Safety and post-hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168-175.
12. Dunn EN, Hariprasad SM, Sheth VS. An overview of the Fovista and rinucumab trials and the fate of anti-PDGF medications. Ophthalmic Surg Lasers Imaging Retina. 2017;48:100-104.
13. Connolly B, Desai A, Garcia CA, Thomas E, Gast MJ. Squalamine lactate for exudative age-related macular degeneration. Ophthalmol Clin North Am. 2006;19:381-391.
14. Bora PS, Hu Z, Tezel TH, et al. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc Natl Acad Sci U S A. 2003;100:2679-2684.
15. Regillo CD. ICON-1 in the treatment of neovascular AMD. Paper presented at: Angiogenesis, Exudation and Degeneration; February 11, 2017; Miami, FL.
16. Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383-392.
17. McHarg S, Clark SJ, Day AJ, Bishop PN. Age-related macular degeneration and the role of the complement system. Mol Immunol. 2015;67:43-50.
18. Yaspan BL, Williams DF, Holz FG, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9:eaaf1443.
19. Diniz B, Thomas P, Thomas B, et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087-5096.



